
���� ��1��������������ɶ�����̼���壻
��2��������������ӵĴ��ڣ�Ӧ���ų�̼������ӵĸ��ţ�
��3��������ɫ��Ӧ����ɫ�ܲ�����������ɫΪ��ɫ����K+��
��4���������������ӡ�̼������ӡ���������Ӷ������ɰ�ɫ������
��� �⣺��ľ���к��п����Լ��Σ���Ҫ�ɷ���K2SO4��K2CO3��KCl����
��1��ȡһ����Һ����������HCl��������ӦCO32-+2H+�TCO2��+H2O����Һ������ɫ���ݲ�����֤������̼������ӣ�
�ʴ�Ϊ������ɫ���ݲ�����
��2��ȡ��һ����Һ��Ϊ����SO42-��Ӧ�ȼӹ��������ų��������ӣ��ټ��Ȼ�����Һ���ɰ�ɫ������Ba2++SO42-�TBaSO4����֤��������������ӣ�
�ʴ�Ϊ�����
��3����ɫ��Ӧ����ɫ�ܲ�����������ɫΪ��ɫ������Һ�к���K+�����Լ���K+��������ɫ��Ӧ��������Ϊ����ɫ�ܲ�����������ɫΪ��ɫ���ʴ�Ϊ������ɫ�ܲ�����������ɫΪ��ɫ��
��4��ȡʣ���һ����Һ�������������������۲쵽�г��������������������ӡ�̼������ӡ���������Ӻ����������ɵİ�ɫ����������֤����Һ��һ�����������ӣ��ʴ�Ϊ����
���� ���⿼�����Ӽ��鼰���ʵļ���ͼ���Ϊ��Ƶ���㣬���ճ������ӵļ��鷽�����ų��Լ��ĸ���Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڲⶨ�к���ʵ������Ҫʹ�õ������У���ƽ����Ͳ���ձ����ζ��ܡ��¶ȼ� | |
| B�� | Ϊ��ȷ�ⶨ��Ӧ�����Һ���¶ȣ�ʵ�����¶ȼ�ˮ����Ӧ��С�ձ��ײ��Ӵ� | |
| C�� | ��50 mL 0.55 mol•L-1��NaOH��Һ��60 mL 0.50 mol•L-1�����ᷴӦ����õ��к�����ֵƫ�� | |
| D�� | ʹ�û��β����������Ϊ�˼Ӵ�Ӧ���ʣ���Сʵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��40% | B�� | ��40% | C�� | =40% | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ԭ����ͭ����Ӧ��ɺ���ֹͣͨ������ֹͣ���� | |
| B�� | ������FeCl3��Һ����������Ʊ�Fe��OH3���壩 | |
| C�� | ��ҵ�Ʊ�HCl����ʱ�������������Ļ�������ڹ��������·�Ӧ | |
| D�� | Ũ����ϡ��ʱ��ͼ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4��g��+$\frac{3}{2}$O2��g���T2H2O��l��+CO��g����H1 | B�� | 2CO��g��+O2��g���T2CO2��g����H2 | ||
| C�� | S��s��+$\frac{3}{2}$O2��g���TSO3��s����H3 | D�� | C6H12O6��s��+6O2��g���T6CO2��g��+6H2O��l����H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
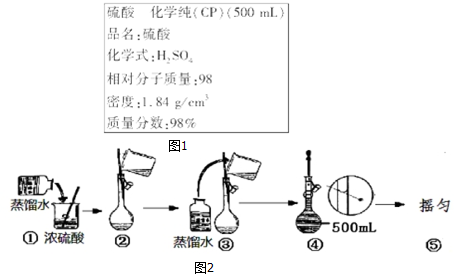
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
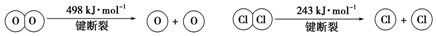
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com