
(11��) �ס��ҡ����������ֵ����ڵ�ȼ������������������X��Y��Z��W���ֻ����ת����ϵ����ͼ��ʾ��

��֪��
�� �ס��ҡ�����Ϊǰ������Ԫ�صĵ��ʣ������¾�Ϊ���壬�����ճ������е�һ�ֳ���������
�� �����£�X����ɫҺ�壬Y�Ǻ�ɫ���塣
�� ��������ȼ�շ�����ɫ�Ļ��棬W��ˮ��Һ�ʻ�ɫ��
��ش�
������д��ѧʽ����_____________�� Z_____________��
����������X�ڸ�����Ҳ�ܵõ�Y����д���÷�Ӧ�Ļ�ѧ����ʽ
___________________________________________________��
��3��������W����Һ���뼸��KSCN��Һ��Ѹ�ٱ�Ϊ��ɫ����д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
��4������������ʵ�Ԫ�ؿ��γ�ԭ�Ӹ�����1��1�Ļ�����H����H����Ʒ�����Һ����ɫ����ȥ�����Ⱥ���ҺΪ ������ɫ����
��5����Yǡ������Z��ˮ��Һ�������еͼ۽������ӵķ����ǵ�������KMnO4����ɫ��ȥ��˵���и����ӣ���ϸ�������ָý��۴���ȱ�ݣ��ˡ�ȱ�ݡ��� ��
(1) O2��HCl
(2) 3Fe + 4H2O Fe3O4 + 4H2
Fe3O4 + 4H2
(3) Fe3��+ 3SCN��= Fe(SCN)3 (4) ��ɫ
(5) �����������£�Cl��Ҳ�ܽ�MnO4����ԭ��ʹ����ɫ
����������1�����ƶ��⣬Ҫ�������ۡ��������Тۿ���Ϊ����㣬��������ȼ�շ�����ɫ�Ļ��棬���Ǻ������ƶϣ���ΪH2����ΪCl2��ZΪHCl��W��ˮ��Һ�ʻ�ɫ������Fe3+��WΪFeCl3���ƶ�Ϊ����Fe�������£�X����ɫҺ�壬XΪH2O�����ΪO2��YΪFe3O4
��2������X�ڸ�����Ҳ�ܵõ�Y�Ļ�ѧ����ʽΪ3Fe +
4H2O Fe3O4 + 4H2
Fe3O4 + 4H2
��3��Fe3����SCN���Ժ�ɫ��������������Fe3�������ӷ���ʽΪFe3��+ 3SCN��= Fe(SCN)3��
��4������������ʵ�Ԫ�ؿ��γ�ԭ�Ӹ�����1��1�Ļ�����H2O2��H2O2����ǿ�����ԣ���ʹƷ����ɫ�����ȣ����ָܻ���������Һ������ɫ��
��5������KMnO4��������Fe2����ͬʱҲ������Cl������������KMnO4��ɫ��ȥ��������֤��Һ��һ������Fe2����


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ɽ��ʡʤ������һ��2012�������ѧ�ڵ�һ�ε��п��Ի�ѧ���� ���ͣ�022
�ס��ҡ�����������ǰ20��Ԫ����ɵ����ʣ�����4������µķ�Ӧ������ת����ϵ���ף��ҡ����������밴Ҫ��ش��������⣺
(1)���Ͷ���ͬ����Ԫ����ɵĵ��ʣ��Ҽ����Ԫ��λ�ڵ������ڣ���Ϊˮ��
������ĵ���ʽΪ________��
�ڸ÷�Ӧ�����ӷ���ʽΪ________��
(2)����Ϊ��̬���ӻ���������������Ԫ�صĺ˵����֮��Ϊ26����Ϊˮ����Ϊ�����ڼȺ��зǼ��Լ��ֺ��м��Լ��Ŀ�ȼ�����壮
������ĽṹʽΪ________��
�ڶ���ϡ��Һ�����ᷴӦ����1 molˮʱ����ΪX kJ����д���˷�Ӧ���Ȼ�ѧ����ʽ________��
(3)�������������ˮ��ҺpH����7����Ϊ����ɫ���壻��Ϊ��ɫ���嵥�ʣ���Ϊ������Σ���������ס������ֻ�������ڼ���ƿ��ǡ����ȫ��Ӧ����ȴ���ٽ��ü���ƿ������ˮ�д����ӣ��۲�ˮ�����뼯��ƿ�У�ʣ������ռ��Ӧǰ�����������1/11��
���ڼ����ҵķ�Ӧ�У�����(���������ԭ��)________�ԣ�
�ڻ����Թ۲쵽����ƿ�е�������________�����з�Ӧ�Ļ�ѧ����ʽΪ________��
(4)����Ϊ����ɫ���嵥�ʣ���Ϊ������������������ϣ���ˮ��ҺpH��7����Ϊ�����Ȼ����ΪHCl��������6.72 L��Cl2(��״����)ʱ�õ�0.10 mol����
�������ӷ���ʽ��ʾ�ҵ�ˮ��ҺpH��7��ԭ��________��
����д���÷�Ӧ�Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���ӿ��ﵽ�������ϡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ���ѡ��
(11��)ij̽��С����ʵ��������������(��Ҫ�ɷ�ΪAl2O3��������Fe2O3��SiO2)��ȡ���������ش��������⣺
(1)��ʵ��������1 mol��L��1��NaOH��Һ480 mL�����Ƹ���Һ��������������������ƽ(����)����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ�������_______________________��
�����ղ������õ����������е�һ�֣���������________��
(2)д��������з�����Ӧ�����ӷ���ʽ____________________________________
________________________________________________________________________��
(3)��������ϴ����β���_______________________________________________
________________________________________________________________________��
(4)��ͬѧ��ʵ������������װ���Ʊ�CO2���壬��ͨ����ҺB����ȡAl(OH)3ʱ�����û�в���Ԥ������
��ͬѧ������Ϊ����ͬѧͨ��CO2�����ǵ���ʵ��ʧ�ܵ�ԭ��֮һ������Ϊ�ҵķ����Ƿ������________�����������������ӷ���ʽ������ԭ��________________________________________________________��
(������Ϊ���������ÿղ�����)
��ͬѧ������Ϊ����ͬѧͨ���CO2�к���HCl���壬Ҳ�ǵ���ʵ��ʧ�ܵ�ԭ����ʵ��������ijװ�ÿɽ��������⡣�������ͬѧ������װ��ͼ����ע���Լ����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��4 3.2ˮ�ĵ������Һ���������ϰ���������棩 ���ͣ�ѡ����
(12��)�мס������ݵ������Ũ�Ⱦ�Ϊ0.1 mol/L�İ�ˮ��pHΪ11.
(1)��������ˮϡ��100������NH3��H2O�ĵ���ƽ�⽫��______(ѡ��ٽ��������ơ�)����ķ����ƶ�����Һ��pH��Ϊ______(ѡ�����)��
A��9��11֮�� B��11
C��12��13֮�� D��13
(2)����0.1 mol/L���Ȼ����Һϡ��100����ϡ�ͺ������Һ��ϡ�ͺ�ļ���Һ��Ƚϣ�pH______(ѡ��״��Ҵ���ȡ�)����ԭ����__________________________
________________________________________________________________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ������ѧ�ڿ�ѧ���Ի�ѧ�Ծ��������棩 ���ͣ������
��11 �֣���֪ A��B��DΪ��ѧ�����ĵ��ʣ��ס��ҡ�����������Ϊ������Ԫ����ɵĻ�������У�����һ����ʹʪ��ĺ�ɫʯ����ֽ��������ɫ���壻����һ�ָ���ȼ�ϣ������Ԫ�������ͬ��1 mol �������в�ͬԭ�ӵ���Ŀ��Ϊ1 ��2���Һ���18 mol���ӣ�����һ��������ˮ�İ�ɫ��״���ʣ�������ǿ�ᷴӦ��Ҳ����ǿ�Ӧ�����о�ˮ���á������ʼ��ת����ϵ����ͼ��ʾ��ijЩ��������ȥ����

��ش𣺣�1������B�����Ԫ�������ڱ��е�λ����_________��
��2����Ļ�ѧʽΪ________������ǿ�Ӧ�����ӷ���ʽ��________________
��3�������������Ļ�ѧ��������________ ������ĸ��ţ���
a�����Ӽ� b�����Թ��ۼ� c���Ǽ��Թ��ۼ�
��4����Ӧ�ٵĻ�ѧ����ʽΪ________________________��
��5����Ӧ���У�0��5mol NaClO�μӷ�Ӧʱ��ת��1 mol���ӣ��仯ѧ����ʽΪ_________
��6�����������£�A��TiO2��C��ʯī����Ӧֻ�����Һ�̼���ѣ�TiC�������߾�ΪijЩ���½ṹ�մɵ���Ҫ�ɷ֡���֪���÷�Ӧ����1 mol��ʱ�ų�536 kJ���������Ȼ�ѧ����ʽΪ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijҽѧ��������ͼ11-9��ʾ�ס����������ʵĽṹ��ʽ�������ֽṹ��ʽ�䲻�Ǻܷ����л���ṹ��ʽ����д�淶���������ܹ�����ر�ʾ�ס����������ʵĽṹ������
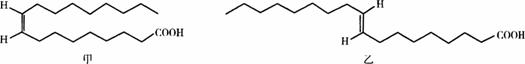
ͼ11-9
���������ѧ���Ļ�ѧ֪ʶ�ش��������⣺
(1)д���ķ���ʽ___________�����ҿɻ���Ϊ___________(�ͬϵ���ͬ���칹�塱)������Ϊ�ס���������һ���Ƿ�ʽ֬����?___________��
(2)����Ϊ�ס������������ܷ��ڿ�����ȼ��?___________��������Ϊ�ܹ�ȼ�գ���д����������������ȼ�յĻ�ѧ����ʽ��_________________________________��
(3)˳ʽ֬�����ΪҺ̬���ռ������״����ʽ֬�����Ϊ��̬���ռ�����͡�ѪҺ�з�ʽ֬���Ậ�����ߣ���ʹ��Һ����Ѫ�ܶ���������Ѫ�ܼ�����ԭ����_________________________��
(4)����֬���⻯(���Ʊ��������͡�����)�����У����ͻᷢ���仯�����⣬��֬��ʱ����¼��ȣ�Ҳ�������ʽ֬���ᡣ��ag��ת����������QkJ������д�����Ȼ�ѧ��Ӧ����ʽ��___________(�á��ס������ҡ���ʾ���ʷ���ʽ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com