
Ķź³ÉĻĀĮŠČČ»Æѧ·½³ĢŹ½£Ø»Æѧ·½³ĢŹ½”¢µē¼«·“Ó¦Ź½”¢±ķ“ļŹ½µČ£©µÄŹéŠ“£ŗ
£Ø1£©ŅŃÖŖ£ŗ2Cu(s)£«1/2O2(g)=Cu2O(s)£»”÷H = -169kJ”¤mol-1£¬
C(s)£«1/2O2(g)=CO(g)£»”÷H = -110.5kJ”¤mol-1£¬
Cu(s)£«1/2O2(g)=CuO(s)£»”÷H = -157kJ”¤mol-1
ÓĆĢæ·ŪŌŚøßĪĀĢõ¼žĻĀ»¹ŌCuOÉś³ÉCu2OµÄČČ»Æѧ·½³ĢŹ½ŹĒ£ŗ
£Ø2£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬¶žŃõ»ÆĮņŗĶŃõĘų·¢ÉśČēĻĀ·“Ó¦£ŗ2SO2(g)+O2(g) 2SO3(g)£¬Š“³öøĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½£ŗ
2SO3(g)£¬Š“³öøĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£Źż±ķ“ļŹ½£ŗ
£Ø3£©ŅŌ¼×Ķ锢æÕĘųĪŖ·“Ó¦Īļ£¬KOHČÜŅŗ×÷µē½āÖŹČÜŅŗ¹¹³ÉČ¼ĮĻµē³Ų£¬Ōņøŗ¼«·“Ó¦Ź½ĪŖ£ŗ ”£
£Ø4£©ĪŽĖ®AlCl3ĘæøĒ“ņæŖÓŠ°×Īķ£¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø5£©”°Ć¾”Ŗ“ĪĀČĖįŃĪ”±Č¼ĮĻµē³Ų£¬Ęä×°ÖĆŹ¾ŅāĶ¼ČēĶ¼£¬øƵē³Ų·“Ó¦µÄ×Ü·“Ó¦·½³ĢŹ½ĪŖ_____________________”£
£Ø6£©¹¤ŅµÉĻµē½ā±„ŗĶŹ³ŃĪĖ®µÄĄė×Ó·½³ĢŹ½ĪŖ________________”£
£Ø12·Ö£©£Ø1£©2CuO(s)£«C(s)= Cu2O(s)£«CO(g)£»”÷H ="+34.5" kJ”¤mol-1 £Ø2·Ö£©
£Ø2£©K= c2(SO3)/ c2(SO2) c (O2) £Ø2·Ö£©
£Ø3£©CH4+10OH”„”Ŗ8e”„=CO32”„+7H2O£¬ £Ø2·Ö£©
£Ø4£©AlCl3£«3H2O Al(OH)3£«3HCl £Ø2·Ö£©
Al(OH)3£«3HCl £Ø2·Ö£©
£Ø5£©Mg+ClO-+ H2O= Cl-+Mg(OH)2 £Ø2·Ö£©
£Ø6£©2Cl”„£«2H2O 2OH”„£«Cl2”ü£«H2”ü £Ø2·Ö£©
2OH”„£«Cl2”ü£«H2”ü £Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŅŃÖŖ£ŗ¢Ł2Cu£Øs£©+1/2O2£Øg£©=Cu2O£Øs£©£»”÷H=-169kJ”¤mol-1£¬
¢ŚC£Øs£©+1/2O2£Øg£©=CO£Øg£©£»”÷H=-110.5kJ”¤mol-1£¬
¢ŪCu£Øs£©+1/2O2£Øg£©=CuO£Øs£©£»”÷H=-157kJ”¤mol-1
ÓÉøĒĖ¹¶ØĀÉæÉÖŖ£¬¢Ł-¢Ū”Į2+¢ŚµĆ2CuO£Øs£©+C£Øs£©=Cu2O£Øs£©+CO£Øg£©£»”÷H=-169kJ”¤mol-1-£Ø-157kJ”¤mol-1£©”Į2=-110.5kJ”¤mol-1="+34.5" kJ”¤mol-1£®
¹Ź“š°øĪŖ£ŗ2CuO£Øs£©+C£Øs£©=Cu2O£Øs£©+CO£Øg£©£»”÷H="+34.5" kJ”¤mol-1£®
£Ø2£©æÉÄę·“Ó¦2SO2£Øg£©+O2£Øg£© 2SO3£Øg£©µÄ»ÆŃ§Ę½ŗā³£Źż
2SO3£Øg£©µÄ»ÆŃ§Ę½ŗā³£Źż
¹Ź“š°øĪŖ£ŗ
£Ø3£©Ōµē³Ųøŗ¼«·¢ÉśŃõ»Æ·“Ó¦£¬¼×ĶéŌŚøŗ¼«·Åµē£¬ŃõĘųŌŚÕż¼«·“Ó¦£¬¼īŠŌĢõ¼žĻĀŃõĘų·ÅµēÉś³ÉĒāŃõøłĄė×Ó£¬Õż¼«µē¼«·“Ó¦Ź½ĪŖ2O2+4H2O+8e£=8OH££¬×ܵĵē³Ų·“Ó¦Ź½ĪŖCH4+2O2+2OH”„=CO32”„+3H2O£¬µē³Ų×Ü·“Ó¦Ź½¼õČ„Õż¼«µē¼«·“Ó¦Ź½æɵĆøŗ¼«µē¼«·“Ó¦Ź½ĪŖCH4+10OH”„-8e”„=CO32”„+7H2O£®
¹Ź“š°øĪŖ£ŗCH4+10OH”„-8e”„=CO32”„+7H2O£®
£Ø4£©AlCl3Ė®½āAlCl3+3H2O Al£ØOH£©3+3HClÉś³ÉHCl£¬ĀČ»ÆĒāÓėæÕĘųÖŠµÄĖ®ÕōĘų³Ź°×Īķ£®
Al£ØOH£©3+3HClÉś³ÉHCl£¬ĀČ»ÆĒāÓėæÕĘųÖŠµÄĖ®ÕōĘų³Ź°×Īķ£®
¹Ź“š°øĪŖ£ŗAlCl3+3H2O Al£ØOH£©3+3HCl£®
Al£ØOH£©3+3HCl£®
£Ø5£©ÓÉĶ¼æÉÖŖĆ¾-“ĪĀČĖįŃĪ”±Č¼ĮĻµē³ŲÖŠMgÓėClO£”¢H2O·“Ӧɜ³ÉCl£ÓėMg£ØOH£©2£¬øƵē³Ų·“Ó¦µÄ×Ü·“Ó¦·½³ĢŹ½ĪŖMg+ClO£+H2O=Cl£+Mg£ØOH£©2£®
¹Ź“š°øĪŖ£ŗMg+ClO£+H2O=Cl£+Mg£ØOH£©2£®
£Ø6£©µē½ā±„ŗĶŹ³ŃĪĖ®Éś³ÉĀČĘų”¢ĒāĘų”¢ĒāŃõ»ÆÄĘ£¬µē½ā±„ŗĶŹ³ŃĪĖ®µÄĄė×Ó·½³ĢŹ½ĪŖ2Cl”„+2H2O 2OH”„+Cl2”ü+H2”ü£®
2OH”„+Cl2”ü+H2”ü£®
¹Ź“š°øĪŖ£ŗ2Cl”„+2H2O 2OH”„+Cl2”ü+H2”ü
2OH”„+Cl2”ü+H2”ü
æ¼µć£ŗÓĆøĒĖ¹¶ØĀɽųŠŠÓŠ¹Ų·“Ó¦ČČµÄ¼ĘĖć£»Ōµē³ŲŗĶµē½ā³ŲµÄ¹¤×÷ŌĄķ£»»ÆŃ§Ę½ŗā³£ŹżµÄŗ¬Ņå


 Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°ŃĆŗ×÷ĪŖČ¼ĮĻæÉĶعżĻĀĮŠĮ½ÖÖĶ¾¾¶£ŗ
Ķ¾¾¶I£ŗC(s) +O2 (g) == CO2(g) ”÷H1<0 ¢Ł
Ķ¾¾¶II£ŗĻČÖĘ³ÉĖ®ĆŗĘų£ŗC(s) +H2O(g) == CO(g)+H2(g) ”÷H2>0 ¢Ś
ŌŁČ¼ÉÕĖ®ĆŗĘų£ŗ2CO(g)+O2 (g) == 2CO2(g) ”÷H3<0 ¢Ū
2H2(g)+O2 (g) == 2H2O(g) ”÷H4<0 ¢Ü
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£© Ķ¾¾¶I·Å³öµÄČČĮæ ( Ģī”°“óÓŚ”±”°µČÓŚ”±»ņ”°Š”ÓŚ”±) Ķ¾¾¶II·Å³öµÄČČĮ攣
£Ø2£© ”÷H1”¢”÷H2”¢”÷H3”¢”÷H4µÄŹżŃ§¹ŲĻµŹ½ŹĒ ”£
£Ø3£©12gĢæ·ŪŌŚŃõĘųÖŠ²»ĶźČ«Č¼ÉÕÉś³ÉŅ»Ńõ»ÆĢ¼£¬·Å³ö110£®35kJČČĮ攣ĘäČČ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ĆŗĢæ×÷ĪŖČ¼ĮĻ²ÉÓĆĶ¾¾¶IIµÄÓŵćÓŠ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)2014Äź10ŌĀ³õ£¬Īķö²ĢģĘų¶ą“ĪĖĮÅ°ŗÓ±±”¢Ģģ½ņ”¢±±¾©µČµŲĒų”£ĘäÖŠ£¬Č¼ĆŗŗĶĘū³µĪ²ĘųŹĒŌģ³ÉæÕĘųĪŪČ¾µÄŌŅņÖ®Ņ»”£
£Ø1£©Ęū³µĪ²Ęų¾»»ÆµÄÖ÷ŅŖŌĄķĪŖ£ŗ2NO(g) + 2CO(g) 2CO2(g)+ N2(g)”£”÷H£¼0
2CO2(g)+ N2(g)”£”÷H£¼0
¢ŁøĆ·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ ”£
¢ŚČōøĆ·“Ó¦ŌŚ¾ųČČ”¢ŗćČŻµÄĆܱÕĢåĻµÖŠ½ųŠŠ£¬ĻĀĮŠŹ¾ŅāĶ¼ÕżČ·ĒŅÄÜĖµĆ÷·“Ó¦ŌŚ½ųŠŠµ½t1Ź±æĢ“ļµ½Ę½ŗāדĢ¬µÄŹĒ £ØĢī“śŗÅ£©”£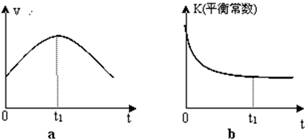
£Ø2£©Ö±½ÓÅÅ·ÅĆŗČ¼ÉÕ²śÉśµÄŃĢĘų»įŅżĘšŃĻÖŲµÄ»·¾³ĪŹĢā”£ĆŗČ¼ÉÕ²śÉśµÄŃĢĘųŗ¬µŖµÄŃõ»ÆĪļ£¬ÓĆCH4“߻ƻ¹ŌNOxæÉŅŌĻū³żµŖŃõ»ÆĪļµÄĪŪČ¾”£
ŅŃÖŖ£ŗCH4(g)+2NO2(g)£½N2(g)£«CO2(g)+2H2O(g) ”÷H£½£867 kJ/mol
2NO2(g)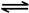 N2O4(g) ”÷H£½£56.9 kJ/mol
N2O4(g) ”÷H£½£56.9 kJ/mol
H2O(g) £½ H2O(l) ¦¤H £½ £44.0 kJ£Æmol
Š“³öCH4“߻ƻ¹ŌN2O4(g)Éś³ÉN2ŗĶH2O(l)µÄČČ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬Ņ²æÉŅŌÓĆNH3“¦ĄķNOx”£ŅŃÖŖNOÓėNH3·¢Éś·“Ӧɜ³ÉN2ŗĶH2O£¬ĻÖÓŠNOŗĶNH3µÄ»ģŗĻĪļ1mol£¬³ä·Ö·“Ó¦ŗóµĆµ½µÄ»¹Ō²śĪļ±ČŃõ»Æ²śĪļ¶ą1.4 g£¬ŌņŌ·“Ó¦»ģŗĻĪļÖŠNOµÄĪļÖŹµÄĮææÉÄÜŹĒ_____________”£
£Ø4£©ŅŌ¼×ĶéĪŖŌĮĻÖĘČ”ĒāĘųŹĒ¹¤ŅµÉĻ³£ÓƵÄÖĘĒā·½·Ø”£Ōņ2 molCH4Óė×ćĮæH2O£Øg£©·“Ó¦×ī¶ąæÉÉś³É_______mol H2£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________________________________________”£
(5)ÉĻŹö·½·ØÖʵƵÄH2æÉŅŌŗĶCOŌŚŅ»¶ØĢõ¼žĻĀŗĻ³É¼×“¼ŗĶ¶ž¼×ĆŃ£ØCH3OCH3£©¼°Šķ¶ąĢžĄąĪļÖŹ”£µ±Į½ÕßŅŌĪļÖŹµÄĮæ1:1“߻Ʒ“Ó¦£¬ĘäŌ×ÓĄūÓĆĀŹ“ļ100%£¬ŗĻ³ÉµÄĪļÖŹæÉÄÜŹĒ ”£
a.ĘūÓĶ b.¼×“¼ c.¼×Č© d.ŅŅĖį
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĆŗµÄĘų»ÆŹĒøߊ§”¢Ēå½ąµŲĄūÓĆĆŗĢæµÄÖŲŅŖĶ¾¾¶Ö®Ņ»”£
(1)ŌŚ250C 101kPaŹ±£¬H2ÓėO2»ÆŗĻÉś³É1mol H2O(g)·Å³ö241.8kJµÄČČĮ棬ĘäČČ»Æѧ·½³ĢŹ½ĪŖ
___________
ÓÖÖŖ: ¢ŁC(s)£«O2(g)ØTCO2(g) ”÷H£½£393.5kJ/mol
¢ŚCO(g)£«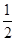 O2(g)ØTCO2(g) ”÷H£½£283.0kJ/mol
O2(g)ØTCO2(g) ”÷H£½£283.0kJ/mol
½¹ĢæÓėĖ®ÕōĘų·“Ó¦ŹĒ½«¹ĢĢåĆŗ±äĪŖĘųĢåČ¼ĮĻµÄ·½·Ø£¬C(s)£«H2O(g)ØTCO(g)£«H2(g) ”÷H=____kJ/mol
(2) COæÉŅŌÓėH2O(g)½ųŅ»²½·¢Éś·“Ó¦: CO(g)£«H2O(g) CO2(g)£«H2(g) ”÷H£¼0ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±n(H2O)=0.20mol£¬n(CO)£½0.10 mol,ŌŚ8000CŹ±“ļµ½Ę½ŗāדĢ¬£¬K£½1.0£¬ŌņĘ½ŗāŹ±£¬ČŻĘ÷ÖŠCOµÄ×Ŗ»ÆĀŹŹĒ_____________(¼ĘĖć½į¹ū±£ĮōŅ»Ī»Š”Źż)”£
CO2(g)£«H2(g) ”÷H£¼0ŌŚŗćČŻĆܱÕČŻĘ÷ÖŠ£¬ĘšŹ¼Ź±n(H2O)=0.20mol£¬n(CO)£½0.10 mol,ŌŚ8000CŹ±“ļµ½Ę½ŗāדĢ¬£¬K£½1.0£¬ŌņĘ½ŗāŹ±£¬ČŻĘ÷ÖŠCOµÄ×Ŗ»ÆĀŹŹĒ_____________(¼ĘĖć½į¹ū±£ĮōŅ»Ī»Š”Źż)”£
(3) ¹¤ŅµÉĻ“ÓĆŗĘų»ÆŗóµÄ»ģŗĻĪļÖŠ·ÖĄė³öH2£¬½ųŠŠ°±µÄŗĻ³É£¬ŅŃÖŖ·“Ó¦·“Ó¦N2(g)£«3H2(g 2NH3(g)£Ø”÷H£¼0£©ŌŚµČČŻĢõ¼žĻĀ½ųŠŠ£¬øıäĘäĖū·“Ó¦Ģõ¼ž£¬ŌŚI”¢II”¢III½×¶ĪĢåĻµÖŠø÷ĪļÖŹÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĻĀĶ¼ĖłŹ¾£ŗ
2NH3(g)£Ø”÷H£¼0£©ŌŚµČČŻĢõ¼žĻĀ½ųŠŠ£¬øıäĘäĖū·“Ó¦Ģõ¼ž£¬ŌŚI”¢II”¢III½×¶ĪĢåĻµÖŠø÷ĪļÖŹÅضČĖꏱ¼ä±ä»ÆµÄĒśĻßČēĻĀĶ¼ĖłŹ¾£ŗ
¢ŁN2µÄĘ½¾ł·“Ó¦ĖŁĀŹv1(N2)”¢vII(N2)”¢vIII(N2)“ӓ󵽊”ÅÅĮŠ“ĪŠņĪŖ________£»
¢ŚÓɵŚŅ»“ĪĘ½ŗāµ½µŚ¶ž“ĪĘ½ŗā£¬Ę½ŗāŅĘ¶ÆµÄ·½Ļņ ŹĒ________£¬²ÉČ”µÄ“ėŹ©ŹĒ________”£
¢Ū±Č½ĻµŚII½×¶Ī·“Ó¦ĪĀ¶Č(T2)ŗĶµŚIII½×¶Ī·“Ó¦ĖŁ¶Č£ØT3)µÄøßµĶ£ŗT2________T3Ģī”°”µ”¢=”¢<”±ÅŠ¶ĻµÄĄķÓÉŹĒ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°±ĘųŹĒÉś²ś»Æ·Ź”¢ĻõĖįµČµÄÖŲŅŖŌĮĻ£¬Ī§ČĘŗĻ³É°±ČĖĆĒ½ųŠŠĮĖŅ»ĻµĮŠµÄŃŠ¾æ
£Ø1£©ĒāĘų¼ČÄÜÓėµŖĘųÓÖÄÜÓėŃõĘų·¢Éś·“Ó¦£¬µ«ŹĒ·“Ó¦µÄĢõ¼žČ“²»ĻąĶ¬”£
ŅŃÖŖ£ŗ2H2 (g) + O2 (g) = 2H2O (g) ¦¤H =" -483.6" kJ/mol
3H2 (g) + N2 (g)  2NH3 (g) ¦¤H =" -92.4" kJ/mol
2NH3 (g) ¦¤H =" -92.4" kJ/mol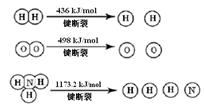
¼ĘĖć¶ĻĮŃ1 mol N”ŌN¼üŠčŅŖÄÜĮæ kJ £¬ µŖĘų·Ö×ÓÖŠ»Æѧ¼ü±ČŃõĘų·Ö×ÓÖŠµÄ»Æѧ¼ü¼ü £ØĢī”°Ēæ”±»ņ”°Čõ”±£©£¬Ņņ“ĖĒāĘųÓė¶žÕß·“Ó¦µÄĢõ¼ž²»Ķ¬”£
£Ø2£©¹ĢµŖŹĒæĘѧ¼ŅÖĀĮ¦ŃŠ¾æµÄÖŲŅŖæĪĢā”£×ŌČ»½ēÖŠ“ęŌŚĢģČ»µÄ“óĘų¹ĢµŖ¹ż³Ģ£ŗN2 (g) + O2 (g) =" 2NO" (g) ¦¤H =" +180.8" kJ/mol £¬¹¤ŅµŗĻ³É°±ŌņŹĒČĖ¹¤¹ĢµŖ”£
·ÖĪöĮ½ÖÖ¹ĢµŖ·“Ó¦µÄĘ½ŗā³£Źż£¬ĻĀĮŠ½įĀŪÕżČ·µÄŹĒ ”£
| ·“Ó¦ | “óĘų¹ĢµŖ | ¹¤Ņµ¹ĢµŖ | ||||
| ĪĀ¶Č/”ę | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84”Į10-31 | 0.1 | 5”Į108 | 1.847 | 0.507 | 0.152 |
 2NH3 (g)²āµĆ¼×ČŻĘ÷ÖŠH2µÄ×Ŗ»ÆĀŹĪŖ40%”£
2NH3 (g)²āµĆ¼×ČŻĘ÷ÖŠH2µÄ×Ŗ»ÆĀŹĪŖ40%”£| | N2 | H2 | NH3 |
| ¼× | 1 | 3 | 0 |
| ŅŅ | 0.5 | 1.5 | 1 |
| ±ū | 0 | 0 | 4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
µŖ¼°Ęä»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²ś”¢Éś»īÖŠÓŠÕßÖŲŅŖ×÷ÓĆ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ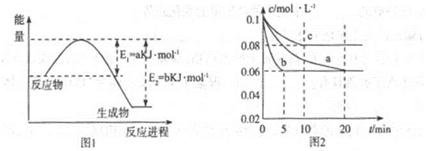
£Ø1£©Ķ¼1ŹĒ1molNO2ŗĶ1molCO·“Ӧɜ³ÉCO2ŗĶNO¹ż³ĢÖŠÄÜŠĒ±ä»ÆŹ¾ŅāĶ¼(a”¢b¾ł“óÓŚ0£¬)ĒŅÖŖ:2CO(g)+2NO(g)=N2(g)+2CO2(g)”÷H=-ckJ”¤mol-1£Øc>0£©
ĒėŠ“³öCO½«NO2»¹ŌÖĮN2Ź±µÄČČ»Æѧ·½³ĢŹ½____________£»
£Ø2£©Ķ¼2ŹĒŹµŃéŹŅŌŚČżøö²»Ķ¬Ģõ¼žµÄĆܱÕČŻĘ÷ÖŠŗĻ³É°±Ź±£¬N2µÄÅضČĖꏱ¼äµÄ±ä»ÆĒśĻß(ŅŌa”¢b”¢c±ķŹ¾£©”£ŅŃÖŖČżøöĢõ¼žĻĀĘšŹ¼¼ÓČėÅØ¶Č¾łĪŖ£ŗc(N2)=0.1mol”¤L-1£¬c(H2)=0.3mol”¤L-1£»ŗĻ³É°±µÄ·“Ó¦£ŗN2(g)+3H2(g) 2NH3(g)”÷H<0
2NH3(g)”÷H<0
¢Ł¼ĘĖćŌŚa“ļĘ½ŗāŹ±H2µÄ×Ŗ»ÆĀŹĪŖ______£»
¢ŚÓÉĶ¼2æÉÖŖ£¬b”¢cø÷ÓŠŅ»øöĢõ¼žÓėa²»Ķ¬£¬ŌņcµÄĢõ¼žøıäæÉÄÜŹĒ______£»
ŹŌŠ“³öÅŠ¶ĻbÓėaĢõ¼ž²»Ķ¬µÄĄķÓÉ____________£»
£Ø3£©ĄūÓĆĶ¼2ÖŠcĢõ¼žĻĀŗĻ³É°±£ØČŻ»ż¹Ģ¶Ø£©”£ŅŃÖŖ»ÆŃ§Ę½ŗā³£ŹżKÓėĪĀ¶Č(T)µÄ¹ŲĻµČēĻĀ±ķ:
¢ŁŹŌČ·¶ØK1µÄĻą¶Ō“󊔣¬K1______4.1x106(ĢīŠ“”°>”±”°-”±»ņ”°<”±£©
¢ŚĻĀĮŠø÷ĻīÄÜ×÷ĪŖÅŠ¶ĻøĆ·“Ó¦“ļµ½»ÆŃ§Ę½ŗāדĢ¬µÄŅĄ¾ŻµÄ ŹĒ______(ĢīŠņŗÅ×ÖÄø£©”£
A£®ČŻĘ÷ÄŚNH3µÄÅØ¶Č±£³Ö²»±ä B£®2v(N2)(Õż£©=v(H2)(Äę£©
C£®ČŻĘ÷ÄŚŃ¹Ēæ±£³Ö²»±ä D£®»ģŗĻĘųĢåµÄĆܶȱ£³Ö²»±ä
£Ø4£©¢ŁNH4ClČÜŅŗ³ŹĖįŠŌµÄŌŅņŹĒ(ÓĆĄė×Ó·“Ó¦·½³ĢŹ½±ķŹ¾ )______”£
¢Ś250CŹ±£¬½«pH=x°±Ė®ÓėpH=yµÄŹčĖį(ĒŅx+y=14,x>11)µČĢå»ż»ģŗĻŗó£¬ĖłµĆČÜŅŗÖŠø÷ÖÖĄė×ÓµÄÅØ¶Č¹ŲĻµÕżČ·µÄŹĒ
A£®[SO42-]>[NH4+]>[H+]>[OH-]
B£®[NH4+]>[SO42-]>[OH-]>[H+]
C£®[NH4+]+[H+]>[OH-]+[SO42-]
D£®[NH4+]>[SO42-]>[H+]>[OH-]
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
æŖ·¢”¢Ź¹ÓĆĒå½ąÄÜŌ“·¢Õ¹”°µĶĢ¼¾¼Ć”±£¬Õż³ÉĪŖæĘѧ¼ŅŃŠ¾æµÄÖ÷ŅŖæĪĢā”£ĒāĘų”¢¼×“¼ŹĒÓÅÖŹµÄĒå½ąČ¼ĮĻ£¬æÉÖĘ×÷Č¼ĮĻµē³Ų”£
£Ø1£©ŅŃÖŖ£ŗ¢Ł 2CH3OH(1) + 3O2(g) = 2CO2(g) + 4H2O(g) ¦¤H1 =" ØC" 1275.6 kJ/mol
¢Ś 2CO(g) + O2(g) = 2CO2(g) ¦¤H2 =" ØC" 566.0 kJ/mol
¢Ū H2O(g) = H2O(1) ¦¤H3 =" ØC" 44.0 kJ/mol
Š“³ö¼×“¼²»ĶźČ«Č¼ÉÕÉś³ÉŅ»Ńõ»ÆĢ¼ŗĶŅŗĢ¬Ė®µÄČČ»Æѧ·½³ĢŹ½£ŗ___________”£
£Ø2£©Éś²ś¼×“¼µÄŌĮĻCOŗĶH2Ą“Ō“ÓŚ£ŗCH4(g) + H2O(g)  CO(g) + 3H2(g) ¦¤H>0
CO(g) + 3H2(g) ¦¤H>0
¢ŁŅ»¶ØĢõ¼žĻĀCH4µÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēĶ¼a”£ŌņTl ________T2(Ģī”°<”±”¢”°>”±”¢”°=”±£¬ĻĀĶ¬)£»A”¢B”¢CČżµć“¦¶ŌÓ¦Ę½ŗā³£Źż£ØKA”¢KB”¢KC£©µÄ“󊔹ŲĻµĪŖ___________”£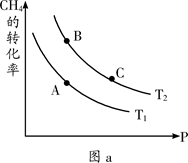
¢Ś100”ꏱ£¬½«1 mol CH4ŗĶ2 mol H2OĶØČėČŻ»żĪŖ1 LµÄ¶ØČŻĆÜ·āČŻĘ÷ÖŠ£¬·¢Éś·“Ó¦£¬ÄÜĖµĆ÷øĆ·“Ó¦ŅŃ¾“ļµ½Ę½ŗāדĢ¬µÄŹĒ__________
a£®ČŻĘ÷ÄŚĘųĢåĆܶČŗć¶Ø
b£®µ„Ī»Ź±¼äÄŚĻūŗÄ0.1 mol CH4Ķ¬Ź±Éś³É0.3 mol H2
c£®ČŻĘ÷µÄŃ¹Ēæŗć¶Ø
d£®3vÕż(CH4) = vÄę(H2)
Čē¹ū“ļµ½Ę½ŗāŹ±CH4µÄ×Ŗ»ÆĀŹĪŖ0.5£¬Ōņ100”ꏱøĆ·“Ó¦µÄĘ½ŗā³£ŹżK =___________
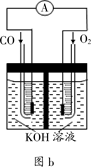
£Ø3£©Ä³ŹµŃ銔×éĄūÓĆCO(g) ”¢ O2(g) ”¢KOH£Øaq£©Éč¼Ę³ÉČēĶ¼bĖłŹ¾µÄµē³Ų×°ÖĆ£¬ŌņøƵē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŃŠ¾æĮņ¼°Ęä»ÆŗĻĪļµÄŠŌÖŹÓŠÖŲŅŖŅāŅ唣
£Ø1£©Cu2SŌŚøßĪĀĢõ¼žĻĀ·¢ÉśČēĻĀ·“Ó¦£ŗ
2Cu2S(s)+3O2(g)=2Cu2O(s)+2SO2(g) ØSH=£773kJ/mol
µ±øĆ·“Ó¦ÓŠ1.2molµē×Ó×ŖŅĘŹ±,·“Ó¦ŹĶ·Å³öµÄČČĮæĪŖ kJ”£
£Ø2£©ĮņĖį¹¤ŅµÉś²śÖŠÉę¼°·“Ó¦£ŗ2SO2(g)+O2(g) 2SO3(g)£¬SO2µÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£
2SO3(g)£¬SO2µÄĘ½ŗā×Ŗ»ÆĀŹÓėĪĀ¶Č”¢Ń¹ĒæµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£
¢ŁŃ¹Ēæ£ŗP1 P2£ØĢī”°£¾”±”¢”°=”±»ņ”°<”±£©”£
¢ŚĘ½ŗā³£Źż£ŗAµć Bµć£ØĢī”°£¾”±”¢”°=”±»ņ”°<”±£©”£
¢Ū200”ęĻĀ£¬½«Ņ»¶ØĮæµÄSO2ŗĶO2³äČėĢå»ż²»±äµÄĆܱÕČŻĘ÷ÖŠ£¬¾10minŗó²āµĆČŻĘ÷ÖŠø÷ĪļÖŹµÄĪļÖŹµÄĮæÅضČČēĻĀ±ķĖłŹ¾:
| ĘųĢå | SO2 | O2 | SO3 |
| ÅØ¶Č£Ømol/L£© | 0.4 | 1.2 | 1.6 |
 ”££ØŅŃÖŖøĆĪĀ¶ČĻĀH2SO3µÄµēĄė³£Źż£ŗKa1=1.0”Į10-2 mol/L£¬Ka2=6.0”Į10-3 mol/L£©
”££ØŅŃÖŖøĆĪĀ¶ČĻĀH2SO3µÄµēĄė³£Źż£ŗKa1=1.0”Į10-2 mol/L£¬Ka2=6.0”Į10-3 mol/L£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
½üÄźĄ“£¬ŅŌĢģČ»ĘųµČĪŖŌĮĻŗĻ³É¼×“¼µÄÄŃĢā±»Ņ»Ņ»¹„æĖ£¬¼«“óµŲ“Ł½ųĮĖ¼×“¼»ÆѧµÄ·¢Õ¹”£
£Ø1£©ÓėĢæŗĶĖ®ÕōĘųµÄ·“Ó¦ĻąĖĘ£¬ŅŌĢģČ»ĘųĪŖŌĮĻŅ²æÉŅŌÖʵĆCOŗĶH2£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________”£
£Ø2£©ŗĻ³É¼×“¼µÄŅ»ÖÖ·½·ØŹĒŅŌCOŗĶH2ĪŖŌĮĻ£¬ĘäÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£ŗ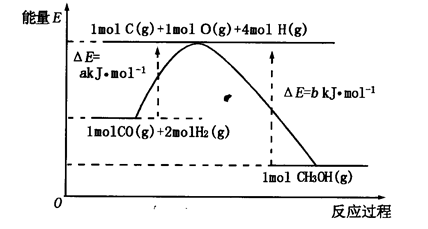
ÓÉĶ¼æÉÖŖ£¬ŗĻ³É¼×“¼µÄČČ»Æѧ·½³ĢŹ½ĪŖ________________________________________”£
£Ø3£©ŅŌCO2ĪŖŌĮĻŅ²æÉŅŌŗĻ³É¼×“¼£¬Ęä·“Ó¦ŌĄķĪŖ£ŗCO2(g)+3H2(g) CH3OH(g)+H2O(g)
CH3OH(g)+H2O(g)
¢ŁŌŚlLµÄĆܱÕČŻĘ÷ÖŠ£¬³äČė1molCO2ŗĶ3molH2£¬ŌŚ500”ęĻĀ·¢Éś·“Ó¦£¬²āµĆCO2(g)ŗĶCH3OH(g)µÄÅضČĖꏱĪŹ±ä»ÆČēĶ¼ĖłŹ¾£ŗ
ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ_________________(Ģī×ÖÄø)£»
| A£®3minŹ±·“Ó¦“ļµ½Ę½ŗā |
| B£®0”«10minŹ±ÓĆH2±ķŹ¾µÄ·“Ó¦ĖŁĀŹĪŖ0£®225mol”¤-1”¤min-1 |
| C£®CO2µÄĘ½ŗā×Ŗ»ÆĀŹĪŖ25£„ |
D£®øĆĪĀ¶ČŹ±»ÆŃ§Ę½ŗā³£ŹżĪŖ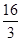 £Ømol/L£©£2 £Ømol/L£©£2 |
| ČŻĘ÷ | ČŻĘ÷1 | ČŻĘ÷2 | ČŻĘ÷3 |
| ·“Ó¦ĪļĶ¶ČėĮæ£ØŹ¼Ģ¬£© | 1molCO2”¢3molH2 | 0.5molCO2”¢1.5molH2 | 1molCH3OH”¢1molH2O |
| CH3OHµÄĘ½ŗāÅضČ/mol?L-1 | c1 | c2 | c3 |
| Ę½ŗāŹ±ĢåĻµŃ¹Ēæ/Pa | p1 | p2 | p3 |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com