
| ×鱚 | c£ØNaOH£©/mol?L-1 | Ź±¼ä/min | |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| 1 | 0.5 | 10.0 | 9.0 | 8.0 | 7.5 | 7.0 | 6.5 | 6.5 | 6.5 |
| 2 | 1.0 | 10.0 | 8.5 | 7.0 | 6.0 | 5.0 | 4.5 | 4.5 | 4.5 |
| 3 | 2.0 | 10.0 | 8.0 | 6.0 | 4.5 | 3.0 | 2.0 | 1.5 | 1.5 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| ×鱚 | C£ØNaOH£©/mol?L-1 | Ź±¼ä/min | ||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 1 | 0.5 | 10.0 | 9.0 | 8.0 | 7.5 | 7.0 | 6.5 | 6.5 | 6.5 | 6.5 |
| 2 | 1.0 | 10.0 | 8.5 | 7.0 | 6.0 | 5.0 | 4.5 | 4.5 | 4.5 | 4.5 |
| 3 | 2.0 | 10.0 | 8.0 | 6.0 | 4.5 | 3.0 | 2.0 | 1.5 | 1.5 | 1.5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ





²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
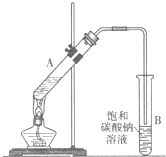 ŅŅĖįŅŅõ„ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£®ŹµŃéŹŅŗĻ³ÉŅŅĖįŅŅõ„µÄ×°ÖĆČēĶ¼ĖłŹ¾£®
ŅŅĖįŅŅõ„ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ£®ŹµŃéŹŅŗĻ³ÉŅŅĖįŅŅõ„µÄ×°ÖĆČēĶ¼ĖłŹ¾£®| ŅŅĖį | ŅŅ“¼ | ŅŅĖįŅŅõ„ | ŅŅĆŃ | |
| ·Šµć/”ę | 118 | 78.3 | 77.1 | 34.5 |
| ČܽāŠŌ | Ņ×ČÜÓŚĖ® | ¼«Ņ×ČÜÓŚĖ® | ÓėŅŅĆŃ»ģČÜ | Ī¢ČÜÓŚĖ® |
| ÅØĮņĖį |
| ”ę140 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŗž±±Ź”ĪäŗŗŹŠøßČż¶žŌĀµ÷ŃŠ²āŹŌĄķæĘ×ŪŗĻ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
ŅŅĖįŅŅõ„ŹĒÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£ŹµŃéŹŅŗĻ³ÉŅŅĖįŅŅõ„µÄ×°ÖĆČēĻĀĶ¼ĖłŹ¾”£

ÓŠ¹ŲŹż¾Ż¼°ø±·“Ó¦:

ø±·“Ó¦£ŗ
C2H5OH+C2H5OH C2H5OC2H5+H2O
C2H5OC2H5+H2O
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚ“óŹŌ¹ÜAÖŠĢķ¼ÓµÄŹŌ¼ĮÓŠ6 mLŅŅ“¼”¢4 mLŅŅĖįŗĶ4 mLÅØĮņĖį,ÕāČżÖÖŹŌ¼ĮµÄĢķ¼ÓĖ³ŠņŅĄ“ĪĪŖ _______”¢_______ ”¢_______
£Ø2£©ŹŌ¹ÜBÖŠµ¼¹Ü½Ó½üŅŗĆęĪ“ÉģČėŅŗĆęĻĀµÄĄķÓÉŹĒ _______
£Ø3£©ĻÖ¶ŌŹŌ¹ÜBÖŠŅŅĖįŅŅõ„“Ö²śĘ·½ųŠŠĢį“棬²½ÖčČēĻĀ£ŗ
¢Ł½«ŹŌ¹ÜBÖŠ»ģŗĻŅŗĢå³ä·ÖÕńµ“ŗó,×ŖČė _______(ĢīŅĒĘ÷Ćū³Ę)½ųŠŠ·ÖĄė;
¢ŚĻņ·ÖĄė³öµÄÉĻ²ćŅŗĢåÖŠ¼ÓČėĪŽĖ®ĮņĖįÄĘ£¬³ä·ÖÕńµ“”£¼ÓČėĪŽĖ®ĮņĖįÄʵÄÄæµÄŹĒ£ŗ
¢Ū½«¾¹żÉĻŹö“¦ĄķµÄŅŗĢå·ÅČėøÉŌļµÄÕōĮóÉÕĘæÖŠ£¬¶ŌĘä½ųŠŠÕōĮó£¬ŹÕ¼Æ_______0C×óÓŅµÄŅŗĢ弓µĆ“æ¾»µÄŅŅĖįŅŅõ„”£
£Ø4£©“ÓĀĢÉ«»ÆѧµÄ½Ē¶Č·ÖĪö£¬Ź¹ÓĆÅØĮņĖįÖĘŅŅĖįŅŅõ„²»×ćÖ®“¦Ö÷ŅŖÓŠ____________________
£Ø5£©³“²ĖŹ±£¬¼ÓŅ»µć°×¾ĘŗĶ“×ÄÜŹ¹²ĖėČĆĮĻćæÉæŚ£¬ŹŌÓĆ·ūŗĻŹµ¼ŹĒéæöµÄ»Æѧ·½³ĢŹ½½āŹĶ: ___ _______________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com