
 +Br2
+Br2| Fe |
 +HBr
+HBr +Br2
+Br2| Fe |
 +HBr
+HBr

 +3Br2��
+3Br2��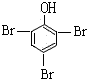 ��+3HBr
��+3HBr +3Br2��
+3Br2�� ��+3HBr
��+3HBr

| 27g |
| 18g/mol |
| 67.2L |
| 22.4L/mol |
| 3mol |
| 0.5mol |
| 1.5mol��2 |
| 0.5mol |
| 27g |
| 18g/mol |
| 67.2L |
| 22.4L/mol |
| 3mol |
| 0.5mol |
| 1.5mol��2 |
| 0.5mol |
 +Br2
+Br2| Fe |
 +HBr��
+HBr�� +Br2
+Br2| Fe |
 +HBr��
+HBr��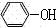 ��
�� +3Br2��
+3Br2�� ��+3HBr��
��+3HBr�� ����ɫ��
����ɫ�� +3Br2��
+3Br2�� ��+3HBr��
��+3HBr�� ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������ʡ��ʦ����2011��2012ѧ��߶���ѧ�ڵ�һ���¿��¿���ѧ���� ���ͣ�038
ij����Һ̬̼�⻯����A������ˮ������²���ɫ��dz���ϲ��Ϊ�Ⱥ�ɫ��0.5 molA��ȫȼ��ʱ���õ�27 g��H2O��67.2 L��CO2(��״��)����ش��������⣮
(1)A�ķ���ʽ________��
(2)A��Һ���ϣ���FeBr3�Ĵ������·�����Ӧ����B��д����Ӧ�ķ���ʽ��________��
(3)B��NaOH��Һ��Ӧ�IJ��ᆳ�ữ��õ��л���C��C�Ľṹ��ʽΪ________����C�м��뱥����ˮ���а�ɫ�����������䷴Ӧ�ķ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ʡ��ʦ���и߶���ѧ�ڵ�һ���¿���ѧ�Ծ����������� ���ͣ������
��8�֣�ij����Һ̬̼�⻯����A������ˮ������²���ɫ��dz���ϲ��Ϊ�Ⱥ�ɫ��0.5molA��ȫȼ��ʱ���õ�27g H2O��67.2L CO2(��״��)����ش��������⡣
��1��A�ķ���ʽ ��
��2��A��Һ���ϣ���FeBr3�Ĵ������·�����Ӧ����B��д����Ӧ�ķ���ʽ��
��
��3��B��NaOH��Һ��Ӧ�IJ��ᆳ�ữ��õ��л���C��C�Ľṹ��ʽΪ ����C�м��뱥����ˮ���а�ɫ�����������䷴Ӧ�ķ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ�ʡ����2���¿������ۣ���ѧ���� ���ͣ������
��16�֣���֪A��B��C��D��E��G��H��I��Ϊ���壬JΪ������Һ̬���ʣ�A��B��C��I��MΪ���ʣ���MΪ���ý�����G��H����ʱ�������̣����Ǵ������µ�ת����ϵ��ͼ�в��ַ�Ӧ��������ʡ�ԣ�����ش��й����⣺

��1��A���ӵĵ���ʽ�� ��G���ӵĿռ乹��Ϊ ��
��2�������£�pHֵ��Ϊ5��H��Һ��K��Һ����ˮ�����c(H+)֮��Ϊ ��
��3������X��ˮ��Һ��ͨ��G�������������� ��N��X�ж���M��Ԫ�أ��仯�ϼ��Ƿ���ͬ ��
��4��д��X+C��Y�����ӷ���ʽ ��
M����̬J�ڸ���ʱ��Ӧ�Ļ�ѧ����ʽ�� ��
��5���������������磬����ͬ����AԪ�ص�Z��Kʩ�õ������Ч�����õ��� - ���ѧʽ����
��6����ͨ��״���£���1 g B������C������ȼ������H����ʱ�ų�92.3 kJ��������2 mol H������ȫ�ֽ�����C�����B������Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱���л������߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com