
�����ճ���������;��㡢�������Ľ������ϡ�
��1�������£�������������ʢװŨ�����ԭ���� ��
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��

��ʪ���������� ���Թ��з�Ӧ�Ļ�ѧ����ʽ�� ��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ
������ȫ�ܽ⣬������Һ�д��ڵ���������_____ ������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����塣���ⶨ�����к���SO2��CO2��H2��
�� ����Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
�� �����л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�� ��672 mL����״�����ռ���������ͨ��������ˮ�У���Ӧ�����ӷ���ʽΪ ��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66 g���ɴ���֪�ռ�����������SO2����������� ��
��1�� Ũ����ʹ�������γ�һ�������ȶ�������Ĥ
��2�����ṩˮ����
3Fe+ 4H2O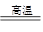 Fe3O4 + 4H2
�� b
Fe3O4 + 4H2
�� b
��3���� 2Fe +
6H2SO4(Ũ)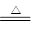 Fe2(SO4)3
+ 3SO2 + 6H2O
Fe2(SO4)3
+ 3SO2 + 6H2O
�� C +2 H2SO4(Ũ)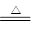 CO2
+2SO2 +2H2O
CO2
+2SO2 +2H2O
�� SO2+Br2+2H2O==2Br- + SO42-+ 4H+ 2/3
����������1�������£�����Ũ�����з����ۻ���Ũ����ʹ�������γ�һ�������ȶ�������Ĥ�����Կ�����������ʢװŨ���ᡣ
��2���ٸ����£�����ˮ������Ӧ��������������ʪ�����������ṩˮ��������Ӧ�ķ���ʽ��3Fe+ 4H2O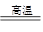 Fe3O4 + 4H2 ��
Fe3O4 + 4H2 ��
�������ڷ�Ӧ�����ǹ����ģ����Է�Ӧ��Ĺ��������У�����������������Ҳ���������������ǹ����ģ�������Һ��һ����Fe2+��H+��������Fe3+����ѡb��
��3����Ũ�������ǿ�����ԣ���������Ũ���ᷴӦ�Ļ�ѧ����ʽ��
2Fe + 6H2SO4(Ũ)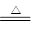 Fe2(SO4)3
+ 3SO2 + 6H2O
Fe2(SO4)3
+ 3SO2 + 6H2O
���������л�ԭ����̼����̼Ҳ�ܺ�Ũ���ᷴӦ������ʽ��C +2 H2SO4(Ũ)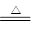 CO2
+2SO2 +2H2O��
CO2
+2SO2 +2H2O��
��SO2�ܰ���ˮ��������Ӧ�ķ���ʽ�� SO2+Br2+2H2O==2Br- + SO42-+ 4H+��4.66g��ɫ���������ᱵ���������ᱵ�����ʵ�����0.02mol����SO2�����ʵ���Ҳ��0.02mol���������0.03mol�����SO2�����������2/3��


 ������ϵ�д�
������ϵ�д� �żӾ���ϵ�д�
�żӾ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����ճ���������;��㡢�������Ľ������ϣ�
�����ճ���������;��㡢�������Ľ������ϣ�
| ||
| ||
| ||
| ||
| ||
| ||
| 2 |
| 3 |
| 2 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ճ���������;��㡢�������Ľ������ϡ�
��1�������£�������������ʢװŨ�����ԭ���� ��
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��
��ʪ���������� ���Թ��з�Ӧ�Ļ�ѧ����ʽ�� ��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ������ȫ�ܽ⣬������Һ�д��ڵ���������_____������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����塣���ⶨ�����к���SO2��CO2��H2��
�� ����Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
�� �����л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�� ��672 mL����״�����ռ���������ͨ��������ˮ�У�������Ӧ��
SO2 + Br2 + 2H2O = 2HBr+ H2SO4��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66 g���ɴ���֪�ռ�����������SO2����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�챱���б�ʦ��ѧУ�����ڶ����¿���ѧ�Ծ����������� ���ͣ������
�����ճ���������;��㡢�������Ľ������ϡ�
��1�������£�������������ʢװŨ�����ԭ���� ��
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��
��ʪ���������� ���Թ��з�Ӧ�Ļ�ѧ����ʽ�� ��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ
������ȫ�ܽ⣬������Һ�д��ڵ���������_____ ������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����塣���ⶨ�����к���SO2��CO2��H2��
������Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
�������л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�۽�672 mL����״�����ռ���������ͨ��������ˮ�У���Ӧ�����ӷ���ʽΪ ��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66 g���ɴ���֪�ռ�����������SO2����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ������һ���¿����գ���ѧ�Ծ��������棩 ���ͣ������
�����ճ���������;��㡢�������Ľ������ϡ�
(1)�����£�������������ʢװŨ�����ԭ���� ��
��2��ijʵ��С��������ͼװ����֤����ˮ�����ķ�Ӧ��
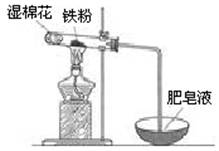
��ʪ���������� ���Թ��з�Ӧ�Ļ�ѧ����ʽ�� ��
��ʵ�������ȡ��������Ӧ��Ĺ������Թ��У�����������ᣬ������ȫ�ܽ⣬������Һ�д��ڵ��������� ������ţ���
a��һ����Fe2+��H+��Fe3+ b��һ����Fe2+��H+��������Fe3+
c��һ����Fe2+��Fe3+�������� H+ d��һ����Fe3+��H+��������Fe2+
��3������ȡһ��������������������Ũ�����У����ȣ���ַ�Ӧ���ռ����塣���ⶨ�����к���SO2��CO2��H2��
�� ����Ũ���ᷴӦ�Ļ�ѧ����ʽ�� ��
�� �����л���CO2��ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
�� ��672 mL����״�����ռ���������ͨ��������ˮ�У�������Ӧ��
SO2 + Br2 + 2H2O = 2HBr + H2SO4��Ȼ���������BaCl2��Һ����ϴ�ӡ�����õ�����4.66 g���ɴ���֪�ռ�����������SO2����������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com