
ΓΨΧβΡΩΓΩΧΦΧΦΥΪΦϋ”–»γœ¬Υυ ΨΒΡΕœΝ―ΖΫ ΫΘΚ
 ΘΜ
ΘΜ
 ΓΘ
ΓΘ
ΗΏΖ÷Ή”ΒΞΧεA(C6H10O3)Ω…Ϋχ––»γœ¬Ζ¥”ΠΘ®Ζ¥”ΠΩρΆΦΘ©

“―÷ΣΘΚ
Δώ.Ε‘ΩρΆΦ÷–Ρ≥–©Μ·ΚœΈο–‘÷ ΒΡΥΒΟςΘΚA‘Ύ “Έ¬œ¬≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§ΒΪΩ…”κNaΖ¥”ΠΖ≈≥ωH2ΘΜBΩ…”κNaHCO3»ή“ΚΖ¥”ΠΖ≈≥ωCO2ΘΜCΩ…”κNaΖ¥”ΠΖ≈≥ωH2ΕχD≤ΜΡήΘΜG‘Ύ “Έ¬œ¬Φ»≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§“≤≤Μ”κNaΖ¥”ΠΖ≈≥ωH2ΓΘ
Δρ.ΝΫΗω“ΜOHΝ§‘ΎΆ§“ΜΗωC‘≠Ή”…œΒΡΫαΙΙ≤ΜΈ»Ε®ΓΘ
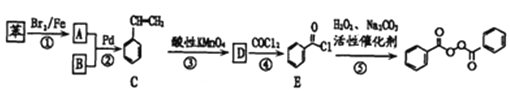
Θ®1Θ©–¥≥ωΖ¥”ΠΔΌ÷–(1)ΒΡΖ¥”Πάύ–ΆΘΚ___________ΘΜ–¥≥ωDΒΡΦϋœΏ ΫΘΚ____________________ΓΘ
Θ®2Θ©–¥≥ωΈο÷ EΥυΚ§ΙΌΡήΆ≈ΒΡΟϊ≥ΤΘΚ ____________________ΓΘ
Θ®3Θ©B‘Ύ≈®H2SO4¥φ‘Ύœ¬”κΦΉ¥ΦΙ≤»»Ζ¥”Π…ζ≥…ΒΡ”–ΜζΈοΒΡœΒΆ≥Οϊ≥ΤΈΣ____________________ΓΘ
Θ®4Θ©–¥≥ωFΓζGΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΘΚ____________________________________ΓΘ
Θ®5Θ©”κBΜΞΈΣΆ§Ζ÷“λΙΙΧεΘ§Ζ÷Ή”ΈΣΝ¥Ή¥ΒΡθΞάύΈο÷ Ι≤”–______________÷÷Θ®≤ΜΩΦ¬«ΝΔΧε“λΙΙΘ©ΓΘ
Θ®6Θ©«κ…ηΦΤΚœάμΖΫΑΗΘ§”ΟΈο÷ E÷Τ±Η““ΕΰΥα(ΤδΥϊ‘≠ΝœΉ‘―ΓΘ§”ΟΖ¥”ΠΝς≥ΧΆΦ±μ ΨΘ§≤ΔΉΔΟς±Ί“ΣΒΡΖ¥”ΠΧθΦΰ) ______________ΓΘάΐ»γΘΚ
![]()
ΓΨ¥πΑΗΓΩ Υ°Ϋβ(Μρ»Γ¥ζ)Ζ¥”Π ![]() τ ΜυΓΔτ»Μυ ΦΉΜυ±ϊœ©ΥαΦΉθΞ
τ ΜυΓΔτ»Μυ ΦΉΜυ±ϊœ©ΥαΦΉθΞ 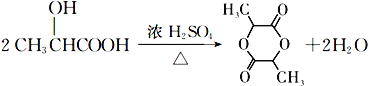 5
5 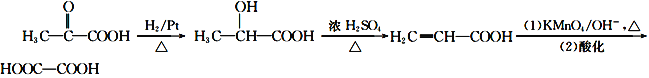
ΓΨΫβΈωΓΩ ‘ΧβΖ÷ΈωΘΚIΘ° “Έ¬œ¬A≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§ΒΪΩ…”κNaΖ¥”ΠΖ≈≥ωH2Θ§ΥΒΟςA÷–≤ΜΚ§τ»ΜυΘ§Κ§”–τ«ΜυΘ§”…Χβ÷–Ζ¥”ΠΧθΦΰΦΑΖ¥”ΠΒΡ≤ζΈοΩ…÷ΣΘ§AΡήΖΔ…ζΥ°ΫβΖ¥”ΠΘ§‘ρA÷–Κ§”–θΞΜυΘΜBΩ…”κNaHCO3»ή“ΚΖ¥”ΠΖ≈≥ωCO2Θ§ΥΒΟςB÷–Κ§”–τ»ΜυΘ§CΩ…”κNaΉς”ΟΖ≈≥ωH2ΕχD≤ΜΡήΘ§ΥΒΟςC «¥ΦΘ§CΖΔ…ζΖ÷Ή”ΦδΆ―Υ°…ζ≥…DΘ§ΗυΨίΖ÷Ή” Ϋ÷ΣΘ§C «““Εΰ¥ΦΘ§DΒΡΫαΙΙΦρ ΫΈΣ![]() ΘΜG‘Ύ “Έ¬œ¬Φ»≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§“≤≤Μ”κNaΉς”ΟΖ≈≥ωH2Θ§ΥΒΟςG÷–≤ΜΚ§τ»ΜυΜρ¥Φτ«ΜυΘ§‘ρ÷ΜΚ§θΞΜυΘ§F÷–Κ§”–¥Φτ«ΜυΚΆτ»ΜυΘΜEΡήΖΔ…ζΦ”≥…Ζ¥”ΠΥΒΟςE÷–Κ§”–≤Μ±ΞΚΆΦϋΘ§ΗυΨίEΒΡΖ÷Ή” ΫΫαΚœΧβΗχ–≈œΔ÷ΣΘ§EΒΡΫαΙΙΦρ ΫΈΣCH3COCOOH Θ§‘ρBΒΡΫαΙΙΦρ ΫΈΣCH2=C(CH3)COOHΘ§AΒΡΫαΙΙΦρ ΫΈΣΘΚ
ΘΜG‘Ύ “Έ¬œ¬Φ»≤Μ”κNaHCO3»ή“ΚΖ¥”ΠΘ§“≤≤Μ”κNaΉς”ΟΖ≈≥ωH2Θ§ΥΒΟςG÷–≤ΜΚ§τ»ΜυΜρ¥Φτ«ΜυΘ§‘ρ÷ΜΚ§θΞΜυΘ§F÷–Κ§”–¥Φτ«ΜυΚΆτ»ΜυΘΜEΡήΖΔ…ζΦ”≥…Ζ¥”ΠΥΒΟςE÷–Κ§”–≤Μ±ΞΚΆΦϋΘ§ΗυΨίEΒΡΖ÷Ή” ΫΫαΚœΧβΗχ–≈œΔ÷ΣΘ§EΒΡΫαΙΙΦρ ΫΈΣCH3COCOOH Θ§‘ρBΒΡΫαΙΙΦρ ΫΈΣCH2=C(CH3)COOHΘ§AΒΡΫαΙΙΦρ ΫΈΣΘΚ![]() ΘΜ
ΘΜ
Θ®1Θ©Ζ¥”ΠΔΌ÷–(1)ΒΡΖ¥”Πάύ–Ά «Υ°Ϋβ(Μρ»Γ¥ζ)Ζ¥”ΠΘΜDΒΡΦϋœΏ ΫΈΣ![]() ΓΘ
ΓΘ
Θ®2Θ©Έο÷ EΥυΚ§ΙΌΡήΆ≈ «τ ΜυΓΔτ»ΜυΓΘ
Θ®3Θ©BΘ®CH2=C(CH3)COOHΘ©‘Ύ≈®H2SO4¥φ‘Ύœ¬”κΦΉ¥ΦΙ≤»»Ζ¥”Π…ζ≥…ΒΡ”–ΜζΈοΒΡœΒΆ≥Οϊ≥ΤΈΣΦΉΜυ±ϊœ©ΥαΦΉθΞΓΘ
Θ®4Θ©FΓζGΒΡΜ·―ßΖ¥”ΠΖΫ≥Χ ΫΈΣ ΓΘ
ΓΘ
Θ®5Θ©”κBΘ®CH2=C(CH3)COOHΘ©ΜΞΈΣΆ§Ζ÷“λΙΙΧεΘ§Ζ÷Ή”ΈΣΝ¥Ή¥ΒΡθΞάύΈο÷ ”–CH2=CH COOCH3ΓΔHCOOCH=CHCH3ΓΔHCOOCH2CH=CH2ΓΔCH3COOCH=CH2ΓΔHCOOC(CH3)=CH2Θ§Ι≤”–5÷÷ΓΘ
Θ®6Θ©”ΟΈο÷ E÷Τ±Η““ΕΰΥαΘ§Ω…“‘œ»”…E”κ«βΤχΦ”≥……ζ≥…2-τ«Μυ±ϊΥαΘ§»ΜΚσ2-τ«Μυ±ϊΥαΖΔ…ζœϊ»ΞΖ¥”ΠΒΟΒΫ±ϊœ©ΥαΘ§ΉνΚσ±ϊœ©ΥαΨ≠ΗΏΟΧΥαΦΊ―θΜ·ΓΔ‘ΌΥαΜ·ΒΟΒΫ““ΕΰΥαΓΘΨΏΧεΚœ≥…¬ΖœΏ»γœ¬ΘΚ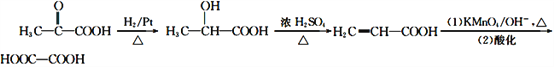 ΓΘ
ΓΘ


 άΦΆΑΌΆ®÷ςΧεΩΈΧΟ–Γ―ßΩΈ ±Ά§≤Ϋ¥ο±ξœΒΝ–¥πΑΗ
άΦΆΑΌΆ®÷ςΧεΩΈΧΟ–Γ―ßΩΈ ±Ά§≤Ϋ¥ο±ξœΒΝ–¥πΑΗ άΦΆΑΌΆ®”≈ΝΖ≤βœΒΝ–¥πΑΗ
άΦΆΑΌΆ®”≈ΝΖ≤βœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ≤ίΥαΨßΧεΒΡΉι≥…Ω…±μ ΨΈΣH2C2O4ΓΛxH2OΘ§Ρ≥―–ΨΩ–‘―ßœΑ–ΓΉι”Οœ¬ΆΦΉΑ÷ΟΫχ––ΓΑ≤ίΥαΨßΧε ή»»Ζ÷ΫβΒΡ≤ΩΖ÷≤ζΈοΒΡ―ι÷ΛΓ±ΒΡ Β―ιΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΓΘ

ΓΨΉ Νœ≤ι‘ΡΓΩ
ΔΌ≤ίΥαΨßΧε‘Ύ101 Γφ ±ΩΣ Φ»έΜ·Θ§150 Γφ ±ΩΣ Φ…ΐΜΣΘ§175 Γφ ±ΩΣ ΦΖ÷ΫβΘΜ
ΔΎ≤ίΥαΗΤΚΆ≤ίΥα«βΗΤΨυΈΣΑΉ…Ϊ≤Μ»ήΈοΓΘ
Θ®1Θ©Α¥’’»γΆΦΥυ ΨΒΡΉΑ÷ΟΘ§Ά®Ιΐ Β―ιΦλ―ι≤ίΥαΨßΧεΒΡ≤ΩΖ÷Ζ÷Ϋβ≤ζΈοΘ§ΉΑ÷ΟB÷–Ω…Ιέ≤λΒΫ”–Τχ≈ίΟΑ≥ω«“≥Έ«ε ·Μ“Υ°±δΜκΉ«Θ§”…¥ΥΦΉΆ§―ß≈–Εœ≤ίΥαΨßΧεΖ÷ΫβΒΡ≤ζΈο÷–”–CO2ΓΘΒΪΝΔΦ¥‘βΒΫ““Ά§―ßΖ¥Ε‘Θ§ΤδΖ¥Ε‘ΒΡάμ”…Ω…Ρή «______________________________________ΓΘ
Θ®2Θ©±ϊΆ§―ß»œΈΣ≤ίΥαΨßΧεΖ÷ΫβΒΡ≤ζΈο÷–Κ§”–COΘ§ΈΣΫχ––―ι÷ΛΘ§X”Π―Γ”Ο________(ΧνΜ·―ß Ϋ)≈®»ή“ΚΘ§ΉΑ÷ΟDΒΡΉς”Ο «____________________ΓΘ
Θ®3Θ© Β―ιΙΐ≥Χ÷–…φΦΑ»γœ¬≤ΌΉςΘΚΔΌΒψ»ΦΉΑ÷ΟA¥ΠΒΡΨΤΨΪΒΤΘΜΔΎœ®ΟπΉΑ÷ΟA¥ΠΒΡΨΤΨΪΒΤΘΜΔέΒψ»ΦΉΑ÷ΟE¥ΠΒΡΨΤΨΪΒΤΘΜΔήœ®ΟπΉΑ÷ΟE¥ΠΒΡΨΤΨΪΒΤΓΘ’β4≤Ϋ≤ΌΉς”…œ»ΒΫΚσΒΡΥ≥–ρΈΣ____________(Χν–ρΚ≈)ΓΘΒψ»ΦE¥ΠΨΤΨΪΒΤ«Α±Ί–κ“ΣΫχ––ΒΡ≤ΌΉς «______________ΓΘ
Θ®4Θ© Β―ιΙΐ≥Χ÷–ΖΔœ÷ΉΑ÷ΟE÷–ΚΎ…ΪΖέΡ©±δΚλ…ΪΘ§ΉΑ÷ΟF÷–”–ΚΎ…ΪΙΧΧε…ζ≥…Θ§Ψ≠Φλ≤βΉΑ÷ΟF÷–ΒΡΙΧΧεΈΣΫπ τΒΞ÷ Θ§‘ρΉΑ÷ΟF÷–ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________________________________________________________________________ΓΘ
Θ®5Θ©ΕΓΆ§―ß”ΟΒΈΕ®Ζ®≤βΕ®≤ίΥαΨßΧε÷–ΫαΨßΥ°ΒΡΚ§ΝΩΘ§Ϋχ––ΝΥœ¬Ν–≤ΌΉςΘΚ
≤Ϋ÷η“ΜΘΚ”ΟΖ÷ΈωΧλΤΫ≥Τ»Γ3.15 g¥ΩΨΜΒΡΗΟ≤ίΥαΨßΧεΘ§≈δ÷Τ≥…250 mL»ή“ΚΓΘ
≤Ϋ÷ηΕΰΘΚ”Ο“Τ“ΚΙή“Τ»Γ25.00 mL¥ΐ≤β≤ίΥα»ή“Κ”ΎΉΕ–ΈΤΩ÷–Θ§≤ΔΦ”»κ ΝΩΝρΥαΥαΜ·ΓΘ
≤Ϋ÷η»ΐΘΚ»Γ0.100 molΓΛLΘ≠1±ξΉΦΥα–‘KMnO4»ή“ΚΘ§Ϋχ––ΒΈΕ®Θ§»ΐ¥ΈΫαΙϊ»γœ¬±μΥυ ΨΘΚ
ΒΎ“Μ¥Έ | ΒΎΕΰ¥Έ | ΒΎ»ΐ¥Έ | |
¥ΐ≤β»ή“ΚΧεΜΐ(mL) | 25.00 | 25.00 | 25.00 |
±ξΉΦ»ή“ΚΧεΜΐ(mL) | 9.99 | 10.01 | 10.00 |
“―÷ΣΒΈΕ®Ζ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚMnO![]() ΘΪH2C2O4ΘΪHΘΪ®DΓζMn2ΘΪΘΪCO2ΓϋΘΪH2O(Έ¥≈δΤΫ)ΓΘ
ΘΪH2C2O4ΘΪHΘΪ®DΓζMn2ΘΪΘΪCO2ΓϋΘΪH2O(Έ¥≈δΤΫ)ΓΘ
ΔΌ≈δ÷Τ≤ίΥα»ή“ΚΒΡ≤ΌΉς≤Ϋ÷η“ά¥Έ «ΘΚΫΪΨßΧε÷Ο”Ύ…’±≠÷–Θ§Φ”Υ°»ήΫβΘ§ΫΪ»ή“ΚΉΣ“Τ»κ________Θ§œ¥Β”Θ§Ε®»ίΘ§“Γ‘»ΓΘ
ΔΎΆ®ΙΐΦΤΥψ»ΖΕ®xΘΫ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡή‘¥ΕΧ»± «»Υάύ…γΜαΟφΝΌΒΡ÷Ί¥σΈ ΧβΓΘΦΉ¥Φ «“Μ÷÷Ω…‘Ό…ζΡή‘¥Θ§ΨΏ”–ΙψΖΚΒΡΩΣΖΔΚΆ”Π”Ο«ΑΨΑΓΘ
Θ®1Θ©ΦΚ÷Σ‘Ύ≥ΘΈ¬≥Θ―Ιœ¬ΘΚ
ΔΌ2CH3OHΘ®lΘ©+3O2Θ®gΘ©=2CO2Θ®gΘ©+4H2OΘ®gΘ©Θ§ΓςH=-1275.6 kJ/mol
ΔΎ2COΘ®gΘ©+O2Θ®gΘ©=2CO2Θ®gΘ©Θ§ ΓςH=-566.0kJ/mol
ΔέH2OΘ®gΘ©=H2OΘ®lΘ©Θ§ ΓςH=-44.0 kJ/mol
–¥≥ωΦΉ¥Φ≤ΜΆξ»Ϊ»Φ…’…ζ≥…“Μ―θΜ·ΧΦΚΆ“ΚΧ§Υ°ΒΡ»»Μ·―ßΖΫ≥Χ Ϋ__________________ΓΘ
Θ®2Θ©Ρ≥ Β―ι–ΓΉι“άΨίΦΉ¥Φ»Φ…’ΒΡΖ¥”Π‘≠άμΘ§…ηΦΤ»γΆΦΥυ ΨΒΡΉΑ÷ΟΓΘ
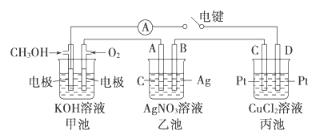
ΔΌΦΉ≥ΊΗΚΦΪΒΡΒγΦΪΖ¥”ΠΈΣ__________________ΓΘ
ΔΎΙΛΉς“ΜΕΈ ±ΦδΚσΘ§≤βΒΟΦΉ÷–»ή“ΚΒΡpHΦθ–ΓΘ§ΗΟΒγ≥ΊΉήΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ______ΓΘ
Δέ““≥Ί÷–AΘ® ·ΡΪΘ©ΒγΦΪΒΡΟϊ≥ΤΈΣ__________________Θ®ΧνΓΑ’ΐΦΪΓ±ΓΔΓΑΗΚΦΪΓ±ΜρΓΑ“θΦΪΓ±ΓΔΓΑ―τΦΪΓ±Θ©Θ§““≥Ί÷–ΉήΖ¥”Π ΫΈΣ_________ΓΘ
ΔήΒ±““≥Ί÷–BΦΪ÷ ΝΩ‘ωΦ”5.40g ±Θ§ΦΉ≥Ί÷–άμ¬έ…œœϊΚΡO2ΒΡΧεΜΐΈΣ__mLΘ®±ξΉΦΉ¥ΩωΘ©Θ§ΦΌ…η““≥ΊΓΔ±ϊ≥Ί÷–ΒΡ»ή“ΚΨυΈΣΉψΝΩΘ§±ϊ≥Ί÷–____Θ®ΧνΓΑCΓ±ΜρΓΑDΓ±Θ©ΦΪΈω≥ω______gΆ≠ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
A.““œ©ΒΡΫαΙΙΦρ Ϋ «CH2CH2
B.““ΥαΚΆ““¥ΦΕΦΦ»Ρή”κΡΤΖ¥”Π≤ζ…ζΤχΧεΘ§”÷Ρή”κΧΦΥαΡΤΖ¥”Π≤ζ…ζΤχΧε
C.C4H10ΒΡ“Μ¬»»Γ¥ζΈοΙ≤”–4÷÷
D.±ξΉΦΉ¥Ωωœ¬Θ§22.4L¬»Ζ¬÷–Κ§”–3molCl‘≠Ή”
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΔΌΜ®…ζ”ΆΚΆΥ°ΜλΚœ“ΚΔΎ39%ΒΡ““¥Φ»ή“ΚΔέ ΙΜκΉ«ΒΡ ≥―ΈΥ°±δ≥Έ«εΘ§Ζ÷άκ…œΗςΜλΚœΈοΒΡ’ΐ»ΖΖΫΖ®“ά¥Έ «Θ® Θ©
A.Ζ÷“ΚΓΔΙΐ¬ΥΓΔ’τΝσ
B.Ιΐ¬ΥΓΔ’τΝσΓΔΖ÷“Κ
C.Ζ÷“ΚΓΔ’τΝσΓΔΙΐ¬Υ
D.’τΝσΓΔΙΐ¬ΥΓΔΖ÷“Κ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ Β―ι “–η“Σ0.1mol/L NaOH»ή“Κ450mLΚΆ0.5mol/LΝρΥα»ή“Κ500mLΘ°ΗυΨί’βΝΫ÷÷»ή“ΚΒΡ≈δ÷Τ«ιΩωΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©»γΆΦΥυ ΨΒΡ“«Τς÷–≈δ÷Τ»ή“ΚΩœΕ®≤Μ–η“ΣΒΡ «______________ΘΜ(Χν–ρΚ≈)Θ§≈δ÷Τ…œ ω»ή“ΚΜΙ–η”ΟΒΫΒΡ≤ΘΝß“«Τς «______________(Χν“«ΤςΟϊ≥Τ)ΘΜ

Θ®2Θ©≈δ÷ΤNaOH ±Θ§‘Ύ Β―ι÷–ΤδΥϊ≤ΌΉςΨυ’ΐ»ΖΘ§»τΕ®»ί ±―ω ”ΩΧΕ»œΏΘ§‘ρΥυΒΟ»ή“Κ≈®Ε»______0.1mol/L(ΧνΓΑ¥σ”ΎΓ±ΓΔΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)Θ°
Θ®3Θ©ΗυΨίΦΤΥψΒΟ÷ΣΘ§Υυ–η÷ ΝΩΖ÷ ΐΈΣ98%ΓΔΟήΕ»ΈΣ1.84g/cm3ΒΡ≈®ΝρΥαΒΡΧεΜΐΈΣ_____________mL(ΦΤΥψΫαΙϊ±ΘΝτ“ΜΈΜ–Γ ΐ)Θ°‘Ύ Β―ι÷–ΤδΥϊ≤ΌΉςΨυ’ΐ»ΖΘ§»τ”ΟΝΩΆ≤ΝΩ»Γ≈®ΝρΥα ±―ω ”ΩΧΕ»œΏΘ§‘ρΥυΒΟ≈δΒΟ»ή“Κ≈®Ε»_____________0.5mol/L(ΧνΓΑ¥σ”ΎΓ±ΓΔΓΑΒ»”ΎΓ±ΜρΓΑ–Γ”ΎΓ±)ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΦΉΓΔ““ΓΔ±ϊΖ÷±π «”…NaΓΔOΓΔH–Έ≥…ΒΡΒΞ÷ Θ§AΓΔBΓΔC «”…HΓΔ0ΓΔNa»ΐ÷÷‘ΣΥΊ÷–ΒΡΝΫ÷÷Μρ»ΐ÷÷Ήι≥…ΒΡΜ·ΚœΈοΘ§Ης÷÷ΒΞ÷ ”κΜ·ΚœΈο÷°Έ ¥φ‘Ύ»γΆΦΥυ ΨΒΡΉΣΜ·–ΠœΒΘΚ

«κΜΊ¥πΘΚ
Θ®1Θ©–¥≥ωœ¬Ν–Έο÷ ΒΡΜ·―ß ΫA________Θ§B_________Θ§C_______ΓΘ
Θ®2Θ©–¥≥ωœ¬Ν–Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
ΔΌΒΞ÷ ΦΉ+Μ·ΚœΈοB_____________________________ΓΘ
ΔΎΜ·ΚœΈοA+Μ·ΚœΈοB___________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΗςΒΊΕ‘ ≥ΤΖΖ«Ζ®ΧμΦ”ΚΆάΡ”ΟΧμΦ”ΦΝΫχ––ΝΥΕύœν’ϊ÷ΈΜνΕ·ΓΘΤδ÷–≥Θ”ΟΒΡΟφΖέ‘ωΑΉΦΝΙΐ―θΜ·±ΫΦΉθΘ( )“≤±ΜΫϊ”ΟΓΘœ¬Οφ «“Μ÷÷“‘±ΫΈΣ‘≠ΝœΚœ≥…Ιΐ―θΜ·±ΫΦΉθΘΒΡΝς≥ΧΘΚ
)“≤±ΜΫϊ”ΟΓΘœ¬Οφ «“Μ÷÷“‘±ΫΈΣ‘≠ΝœΚœ≥…Ιΐ―θΜ·±ΫΦΉθΘΒΡΝς≥ΧΘΚ
“―÷ΣΘΚ![]()
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΙΛ“Β…œΈο÷ B÷ς“Σά¥‘¥”Ύ_____Θ§Έο÷ DΒΡΟϊ≥Τ «_______ΘΜΟΩΗωCΖ÷Ή”÷–ΉνΕύ”–____Ηω‘≠Ή”Ι≤ΤΫΟφΓΘ
Θ®2Θ©ΫαΚœΙΐ―θΜ·±ΫΦΉθΘΫαΙΙΖ÷ΈωΘ§Ιΐ―θΜ·±ΫΦΉθΘΩ…“‘ΉςΈΣ‘ωΑΉΦΝΒΡ‘≠“ρ «________ΓΘ
Θ®3Θ©–¥≥ωΖ¥”ΠΔΎΒΡΜ·―ßΖΫ≥Χ Ϋ________Θ§Ζ¥”Πάύ–ΆΈΣ________ΓΘ
Θ®4Θ©Ρ≥Έο÷ FΈΣΙΐ―θΜ·±ΫΦΉθΘΒΡΆ§Ζ÷“λΙΙΧεΘ§Ά§ ±ΖϊΚœœ¬Ν–ΧθΦΰΒΡFΒΡΆ§Ζ÷“λΙΙΧε”–______÷÷Θ§«κ–¥≥ωΤδ÷–”–»ΐ÷÷≤ΜΆ§Μ·―ßΜΖΨ≥ΒΡ«β‘≠Ή”ΒΡΫαΙΙΦρ Ϋ:____________ΓΘ
ΔΌ Κ§”–ΝΣ±ΫΘ®![]() Θ©ΫαΙΙΒΞ‘Σ.ΈόΤδΥϊΜΖΉ¥ΫαΙΙ
Θ©ΫαΙΙΒΞ‘Σ.ΈόΤδΥϊΜΖΉ¥ΫαΙΙ
ΔΎ ‘Ύ“ΜΕ®ΧθΦΰœ¬ΡήΖΔ…ζ“χΨΒΖ¥”Π
Δέ 1mol FΉνΕύΩ…œϊΚΡ4mol NaOH
Θ®5Θ©«κ–¥≥ω“‘±Ϋ““œ©ΈΣ‘≠ΝœΘ§Κœ≥…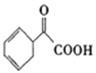 ΒΡΝς≥ΧΘ§ΈόΜζ ‘ΦΝ»Έ―ΓΘ§ΉΔΟςΖ¥”ΠΧθΦΰΓΘ____________
ΒΡΝς≥ΧΘ§ΈόΜζ ‘ΦΝ»Έ―ΓΘ§ΉΔΟςΖ¥”ΠΧθΦΰΓΘ____________
Ψάΐ»γœ¬ΘΚ![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœρΥΡ÷Μ Δ”–œύΆ§ΝΩNaOH»ή“ΚΒΡ…’±≠÷–Ά®»κ≤ΜΆ§ΝΩΒΡCO2ΤχΧεΘ§‘ΎΥυΒΟ»ή“Κ÷–÷πΒΈΦ”»κœΓ―ΈΥα÷ΝΙΐΝΩΘ§≤ΔΫΪ»ή“ΚΦ”»»Θ§≤ζ…ζΒΡCO2ΤχΧε”κHClΈο÷ ΒΡΝΩΒΡΙΊœΒ»γΆΦΘΚΘ®Κω¬‘CO2ΒΡ»ήΫβΚΆHClΒΡΜ”ΖΔΘ©Θ§‘ρœ¬Ν–Ζ÷ΈωΕΦ’ΐ»ΖΒΡΉιΚœ «

Ε‘”ΠΆΦœσ | »ή“Κ÷–ΒΡ÷ς“Σ≥…Ζ÷ | |
A | Δώ | NaOHΓΔNaHCO3 |
B | Δρ | NaHCO3ΓΔNa2CO3 |
C | Δσ | NaOHΓΔNa2CO3 |
D | Δτ | Na2CO3 |
A. A. B. B C. C D. D
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com