


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
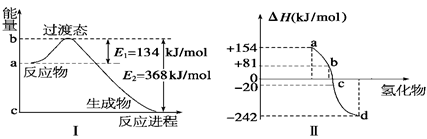
 2Fe(s)+3CO2(g) ��H="-a" kJ/mol
2Fe(s)+3CO2(g) ��H="-a" kJ/mol 2Fe3O4(s)+CO2(g) ��H="-b" kJ/mol
2Fe3O4(s)+CO2(g) ��H="-b" kJ/mol  3FeO(s)+CO2(g) ��H="+ckJ/mol"
3FeO(s)+CO2(g) ��H="+ckJ/mol"  Fe(s)+CO2(g) ��H=- ��
Fe(s)+CO2(g) ��H=- ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��������Ϊ679kJ |
| B��������������Ϊ431kJ |
| C��������������Ӧ����2 mol�Ȼ������壬��Ӧ�ų�183 kJ��mol-1���� |
| D��������������Ӧ����2 mol�Ȼ������壬��Ӧ����183 kJ��mol-1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����֪2H2(g)��O2(g)��2H2O(l)����H����483.6 kJ��mol��1����������ȼ����Ϊ483.6 kJ��mol��1 |
| B����֪C(ʯī��s)��C(���ʯ��s)����H��0������ʯ��ʯī�ȶ� |
| C����֪2C(s)��2O2(g)��2CO2(g)����H1��2C(s)��O2(g)��2CO(g)����H2����H1����H2 |
| D����֪Ni(CO)4(s)��Ni(s)��4CO(g)����H��+Q kJ��mol��1����Ni(s)��4CO(g)��Ni(CO)4(s)����H����Q kJ��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
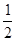 O2(g) �� H2O(g)����H1��a kJ��mol-1?
O2(g) �� H2O(g)����H1��a kJ��mol-1?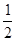 O2(g) �� H2O(l) ��H2��b kJ��mol-1??
O2(g) �� H2O(l) ��H2��b kJ��mol-1??| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ��mol��1 | 436 | 391 | 945 |
 2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a ��ֵ�� (д�� + ��)��
2NH3(g) ��H��a kJ��mol��1���Ը��ݱ������м������ݹ���a ��ֵ�� (д�� + ��)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
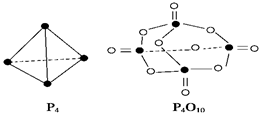
| A����6a+5d-4c-12b�� kJ��mol-1 |
| B����4c +12b -6a-5d�� kJ��mol-1 |
| C����4c +12b -4a-5d�� kJ��mol-1 |
| D����4a+5d-4c -12b�� kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 O2��g����
O2��g���� P4O10��s�� ��H����738.5kJ��mol��1
P4O10��s�� ��H����738.5kJ��mol��1 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
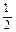 O2(g) =" CO" (g)
O2(g) =" CO" (g) 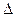 H1= ��110.5kJ/mol
H1= ��110.5kJ/mol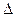 H2= ��393.5kJ/mol
H2= ��393.5kJ/mol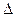 HΪ
HΪ| A��+ 283.5kJ/mol | B��+ 172.5kJ/mol |
| C����172.5kJ/mol | D����504kJ/mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com