
ΓΨΧβΡΩΓΩΒΈΕ® Β―ι «Μ·―ß―ßΩΤ÷–÷Ί“ΣΒΡΕ®ΝΩ Β―ιΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©ΥαΦν÷–ΚΆΒΈΕ®ΓΣΓΣ”Ο≈®Ε»ΈΣ0.1000molΓΛLΘ≠1ΒΡ±ξΉΦ―ΈΥαΒΈΕ®Έ¥÷Σ≈®Ε»ΒΡNaOH»ή“ΚΘ§±μΗώ÷–Φ«¬ΦΝΥ Β―ι ΐΨίΘΚ
ΒΈΕ®¥Έ ΐ | ¥ΐ≤β“ΚΧεΜΐ(mL) | ±ξΉΦ―ΈΥαΧεΜΐ(mL) | |
ΒΈΕ®«ΑΕΝ ΐ | ΒΈΕ®ΚσΕΝ ΐ | ||
ΒΎ“Μ¥Έ | 20.00 | 0.50 | 20.40 |
ΒΎΕΰ¥Έ | 20.00 | 3.00 | 23.00 |
ΒΎ»ΐ¥Έ | 20.00 | 4.00 | 24.10 |
ΔΌœ¬Ν–≤ΌΉς‘λ≥…≤βΕ®ΫαΙϊΤΪΗΏΒΡ «________(Χν―ΓœνΉ÷ΡΗ)
AΘ°ΒΈΕ®÷’ΒψΕΝ ΐ ±Θ§Η© ”ΒΈΕ®ΙήΩΧΕ»Θ§ΤδΥϊ≤ΌΉς’ΐ»ΖΓΘ
BΘ° ΔΉΑΈ¥÷Σ“ΚΒΡΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΙΐΘ§Έ¥”ΟΈ¥÷Σ“Κ»σœ¥
CΘ°Υα ΫΒΈΕ®Ιή”Ο’τΝσΥ°œ¥ΨΜΚσΘ§Έ¥”Ο±ξΉΦ―ΈΥα»σœ¥
DΘ°ΒΈΕ®«ΑΘ§ ΔΉΑ±ξΉΦ“ΚΒΡΒΈΕ®ΙήΦβΉλ”–Τχ≈ίΘ§ΒΈΕ®ΚσΤχ≈ίœϊ ß
ΔΎΗΟNaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ_____________mol/LΓΘΘ®–Γ ΐΒψΚσ±ΘΝτΥΡΈΜ”––ß ΐΉ÷Θ©
Θ®2Θ©―θΜ·ΜΙ‘≠ΒΈΕ®ΓΣΓΣ»Γ“ΜΕ®ΝΩΒΡ≤ίΥαΘ®H2C2O4Θ©»ή“Κ÷Ο”ΎΉΕ–ΈΤΩ÷–Θ§Φ”»κ ΝΩœΓΝρΥαΘ§”Ο±ξΉΦΥα–‘ΗΏΟΧΥαΦΊ»ή“ΚΒΈΕ®ΓΘΒΈΕ® ±KMnO4»ή“Κ”ΠΉΑ‘Ύ______________(ΧνΓΑΥαΓ±ΜρΓΑΦνΓ±) ΫΒΈΕ®Ιή÷–Θ§ΒΈΕ®÷’Βψ ±ΒΈΕ®œ÷œσ «_________________________________ΓΘ
Θ®3Θ©≥ΝΒμΒΈΕ®®D®DΒΈΕ®ΦΝΚΆ±ΜΒΈΕ®ΈοΒΡ…ζ≥…Έο±»ΒΈΕ®ΦΝ”κ÷Η ΨΦΝΒΡ…ζ≥…ΈοΗϋΡ―»ήΓΘ≤ΈΩΦ±μ÷–ΒΡ ΐΨίΘ§»τ”ΟAgNO3ΒΈΕ®NaSCN»ή“ΚΘ§Ω…―Γ”ΟΒΡ÷Η ΨΦΝ «______(Χν―ΓœνΉ÷ΡΗ)ΓΘ
Ρ―»ήΈο | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
―’…Ϊ | ΑΉ | «≥ΜΤ | ΑΉ | Ή©Κλ | ΑΉ |
Ksp | 1.77ΓΝ10Θ≠10 | 5.35ΓΝ10Θ≠13 | 1.21ΓΝ10Θ≠16 | 1.12ΓΝ10Θ≠12 | 1.0ΓΝ10Θ≠12 |
AΘ°NaClBΘ°NaBrCΘ°NaCNDΘ°Na2CrO4
ΓΨ¥πΑΗΓΩCD 0.1000mol/L Υα ΒΈ»κΉνΚσ“ΜΒΈ±ξΉΦ“ΚΚσΘ§ΉΕ–ΈΤΩ÷–»ή“Κ”…Έό…Ϊ±δΈΣΉœΚλ…ΪΘ§«“ΑκΖ÷÷”ΡΎ≤ΜΆ …Ϊ D
ΓΨΫβΈωΓΩ
(1)ΔΌAΘ°ΒΈΕ®÷’ΒψΕΝ ΐ ±Θ§Η© ”ΒΈΕ®ΙήΩΧΕ»Θ§ΒΦ÷¬ΕΝ»ΓΒΡ±ξΉΦ“ΚΧεΜΐΤΪ–ΓΘ§≤βΕ®ΫαΙϊΤΪ–ΓΘ§≤ΜΖϊΚœΧβ“βΘΜ
BΘ° ΔΉΑΈ¥÷Σ“ΚΒΡΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΙΐΘ§Έ¥”ΟΈ¥÷Σ“Κ»σœ¥≤ΜΜα”ΑœλœϊΚΡΒΡ±ξΉΦ“ΚΧεΜΐΘ§Υυ“‘Ε‘ΫαΙϊΈό”ΑœλΘ§≤ΜΖϊΚœΧβ“βΘΜ
CΘ°Υα ΫΒΈΕ®Ιή”Ο’τΝσΥ°œ¥ΨΜΚσΘ§Έ¥”Ο±ξΉΦ―ΈΥα»σœ¥Θ§ΫΪΜαœΓ Ά±ξΉΦ“ΚΘ§ΒΦ÷¬ΕΝ»ΓΒΡ±ξΉΦ“ΚΧεΜΐΤΪ¥σΘ§≤βΕ®ΫαΙϊΤΪΗΏΘ§ΖϊΚœΧβ“βΘΜ
DΘ°ΒΈΕ®«ΑΘ§ ΔΉΑ±ξΉΦ“ΚΒΡΒΈΕ®ΙήΦβΉλ”–Τχ≈ίΘ§ΒΈΕ®ΚσΤχ≈ίœϊ ßΘ§‘ρΕΝ»ΓΒΡ±ξΉΦ“ΚΧεΜΐΤΪ¥σΘ§≤βΕ®ΫαΙϊΤΪ¥σΘ§ΖϊΚœΧβ“βΘΜ
Ήέ…œΥυ ω―ΓCDΘΜ
ΔΎΗυΨί±μΗώΘ§ΒΎ“Μ¥ΈœϊΚΡ±ξΉΦ“ΚΧεΜΐΈΣ20.40mL-0.50mL=19.90mLΘ§ΒΎΕΰ¥ΈœϊΚΡ±ξΉΦ“ΚΧεΜΐΈΣ23.00mL-3.00mL=20.00mLΘ§ΒΎ»ΐ¥ΈœϊΚΡ±ξΉΦ“ΚΧεΜΐΈΣ24.10mL-4.00mL=20.10mLΘ§Υυ“‘»ΐ¥ΈΒΈΕ®œϊΚΡΒΡHCl±ξΉΦ“ΚΤΫΨυΧεΜΐΈΣ20.00mLΘ§‘ρNaOH»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣc=![]() =0.1000mol/LΘΜ
=0.1000mol/LΘΜ
(2)Υα–‘ΗΏΟΧΥαΦΊ»ή“Κœ‘Υα–‘Θ§«“ΨΏ”–«Ω―θΜ·–‘Θ§Υυ“‘ΉΑ‘ΎΥα ΫΒΈΕ®Ιή÷–ΘΜΒΈΕ®÷’Βψ ±Υα–‘ΗΏΟΧΥαΦΊ»ή“ΚΙΐΝΩΘ§»ή“Κ±δΈΣΉœΚλ…ΪΘ§Υυ“‘œ÷œσΈΣΘΚΒΈ»κΉνΚσ“ΜΒΈ±ξΉΦ“ΚΚσΘ§ΉΕ–ΈΤΩ÷–»ή“Κ”…Έό…Ϊ±δΈΣΉœΚλ…ΪΘ§«“ΑκΖ÷÷”ΡΎ≤ΜΆ …ΪΘΜ
(3)»τ”ΟAgNO3»ΞΒΈΕ®NaSCN»ή“ΚΘ§Ω…―Γ”ΟΒΡΒΈΕ®÷Η ΨΦΝΒΡΈο÷ ΒΡ»ήΫβΕ»”Π±»AgSCN¥σΘ§AgClΓΔAgBrΓΔAgCNΚΆAgSCNΨυΈΣΆ§άύ–Ά≥ΝΒμΘ§÷Μ”–AgClΒΡ»ήΕ»Μΐ±»AgSCN¥σΘ§ΒΪAgClΆ§ΈΣΑΉ…Ϊ≥ΝΒμΘ§œ÷œσ≤ΜΟςœ‘Θ§Υυ“‘»ΐ÷÷≥ΝΒμœύ”ΠΒΡ―ΈΨυ≤ΜΚœ Θ§Ag2CrO4”κAgSCN»ήΕ»Μΐœύ≤ν≤Μ¥σΘ§ΒΪAg2CrO4ΈΣA2B–Ά≥ΝΒμΘ§Υυ“‘Ag2CrO4ΒΡ»ήΫβΕ»“Σ±»AgSCNΒΡ»ήΫβΕ»–ΓΘ§«“ΈΣΉ©Κλ…ΪΘ§œ÷œσΟςœ‘Θ§Υυ“‘Ω…“‘―ΓNa2CrO4Ής÷Η ΨΦΝΘ§Φ¥―ΓDΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν– Β―ιΫα¬έ”κ Β―ι≤ΌΉςΦΑœ÷œσ≤ΜœύΖϊΒΡ“ΜΉι «Θ® Θ©
―Γœν | Β―ι≤ΌΉςΦΑœ÷œσ | Β―ιΫα¬έ |
A | œρΡ≥Υα”ξ―υΤΖ÷–Φ”»κBa(OH)2»ή“ΚΘ§”–ΑΉ…Ϊ≥ΝΒμ…ζ≥… | Υα”ξ ‘―υ÷–“ΜΕ®Κ§SO42- |
B | œρKI-ΒμΖέ»ή“Κ÷–ΒΈ»κ¬»Υ°Θ§»ή“Κ±δ≥…άΕ…Ϊ | I-ΒΡΜΙ‘≠–‘«Ω”ΎCl- |
C | ΫΪBa(OH)2ΓΛ8H2OΚΆNH4ClΨßΧε‘Ύ–Γ…’±≠÷–ΜλΚœΫΝΑηΘ§”Ο ÷¥ΞΟΰ…’±≠Άβ±ΎΗ–Ψθ±δΝΙ | Ba(OH)2ΓΛ8H2O”κNH4ClΒΡΖ¥”Π «Έϋ»»Ζ¥”Π |
D | œρΡ≥―Έ»ή“Κ÷–Φ”»κNaOH»ή“ΚΘ§Φ”»»Θ§”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΖ≈‘Ύ ‘ΙήΩΎΘ§ ‘÷Ϋ±δάΕ | ΗΟ―Έ»ή“Κ÷–Κ§”–NH4+ |
A.AB.BC.CD.D
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΜ·―ß”κ»Υάύ…ζΜνΟή«–œύΙΊΓΘ«κΑ¥“Σ«σΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©¥Κ«οΡ©ΤΎΙΛ“’ΙΌ ιΓΕΩΦΙΛΦ«ΓΖ÷–Φ«‘Ί”–ΓΑδ≥≤·Γ±ΒΡΖΫΖ®Θ§Φ¥άϊ”ΟΚ§”–ΧΦΥαΡΤΒΡΥ°»ή“Κά¥œ¥Β”ΥΩ≤·ΓΘ«κ–¥≥ωΧΦΥαΡΤΥ°»ή“Κ÷–Ά®»κCO2ΤχΧεΒΡΜ·―ßΖΫ≥Χ Ϋ____Θ§ΫΪ54.8g Na2CO3ΚΆNaHCO3 ΒΡΜλΚœΈοΖ÷≥…Β»ΝΩΒΡΝΫΖίΘ§“ΜΖί»ή”ΎΥ°ΚσΦ”»κΉψΝΩ―ΈΥαΘ§ ’Φ·ΒΫΤχΧεV LΘ§Νμ“ΜΖί÷±Ϋ”Φ”»»÷ΝΚψ÷ΊΘ§…ζ≥…ΤχΧε2.24LΘ®Υυ”–ΤχΧεΧεΜΐΨυ‘Ύ±ξΉΦΉ¥Ωωœ¬≤βΕ®Θ©Θ§‘ρ‘≠ΙΧΧεΜλΚœΈο÷–Na2CO3ΒΡΈο÷ ΒΡΝΩΘΚnΘ®Na2CO3Θ©ΘΫ____Θ§ΤχΧεVΘΫ____ΓΘ
Θ®2Θ©ΓΑ84Γ±œϊΕΨ“Κ‘Ύ…ζΜν÷– Ι”ΟΙψΖΚΘ§Τδ”––ß≥…Ζ÷ «¥Έ¬»ΥαΡΤΓΘΩ…‘Ύ≥ΘΈ¬œ¬ΫΪ¬»ΤχΆ®»κNaOH»ή“Κ÷ΤΒΟΘ§ΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____Θ§»τ”–2mol¬»Τχ≤Έ”κΗΟΖ¥”ΠΘ§‘ρ¥Υ ±ΉΣ“ΤΒΡΒγΉ” ΐΈΣ____NAΓΘ
Θ®3Θ©–ΓΥ’¥ρΩ…”Ο”Ύ÷ΈΝΤΈΗΥαΙΐΕύΘ§ΤδΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____ΓΘ
Θ®4Θ©≥Λ · «ΒΊ±μ―“ ·Ήν÷Ί“ΣΒΡ‘λ―“ΩσΈοΓΘΡ≥÷÷≥Λ ·ΒΡΜ·―ßΉι≥…KAlSi3O8‘ρΫΪΤδΗΡ–¥≥…―θΜ·ΈοΒΡΉιΚœ–Έ ΫΈΣ____ΓΘ
Θ®5Θ©ΤœΧ―Χ«(Ζ÷Ή” ΫC6H12O6) «»ΥΧεΜνœΗΑϊΒΡΡήΝΩά¥‘¥ΓΘ“―÷Σ1molΒ»”Ύ1000mmolΘ§Ρ≥ΧεΦλΒΞΒΡ“Μ–©÷Η±ξ»γΆΦΘ§‘ρΟΩ…ΐΗΟ―υΤΖ÷–Κ§ΤœΧ―Χ«ΒΡ÷ ΝΩΈΣ____gΘ®«κ±ΘΝτΝΫΈΜ–Γ ΐΘ©ΓΘ
9 | ΑΉ«ρ±» | 1.6 | |
10 | »ιΥαΆ―«βΟΗ | 161 | U/L |
11 | ΝΉΥαΦΓΥαΦΛΟΗ | 56 | U/L |
12 | Η ”Ά»ΐθΞ | 0.52 | mmol/L |
13 | ΉήΒ®ΙΧ¥Φ | 4.27 | mmol/L |
14 | ΗΏΟήΕ»÷§ΒΑΑΉΒ®ΙΧ¥Φ | 1.57 | mmol/L |
15 | ΒΆΟήΕ»÷§ΒΑΑΉΒ®ΙΧ¥Φ | 1.40 | mmol/L |
16 | ΤœΧ―Χ« | 4.94 | mmol/L |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΑ¥“Σ«σΜΊ¥πœ¬Ν–Έ ΧβΓΘ
I.Έε÷÷‘ΣΥΊΒΡ‘≠Ή”ΒγΉ”≤ψΫαΙΙ»γœ¬ΘΚAΘΚ1s22s22p63s23p63d54s2ΓΔBΘΚ1s22s22p63s2ΓΔCΘΚ1s22s22p6ΓΔDΘΚ1s22s22p63s23p2ΓΔEΘΚ[Ar]4s1ΓΘ
«κΜΊ¥πΘΚ(Χν‘ΣΥΊΖϊΚ≈)
Θ®1Θ©________‘ΣΥΊ «œΓ”–ΤχΧεΓΘΚ§Έ¥≥…Ε‘ΒγΉ” ΐΉνΕύΒΡ‘ΣΥΊ «________ΓΘ
Θ®2Θ©A‘ΣΥΊ‘≠Ή”ΒΡΚΥΆβΒγΉ”Ι≤”–________÷÷‘ΥΕ·Ή¥Χ§Θ§ΡήΝΩΉνΗΏΒΡΡήΦΕ «________Θ®ΧνΡήΦΕΖϊΚ≈Θ©ΓΘ
Θ®3Θ©D‘ΣΥΊ‘≠Ή”ΒΡΦέ≤ψΒγΉ”≈≈≤ΦΆΦ «________ΓΘ
Θ®4Θ©________‘ΣΥΊΒΡΒγΗΚ–‘Ήν¥σΘ§________‘ΣΥΊ‘≠Ή”ΒΡΒΎ“ΜΒγάκΡήΉν¥σΘ§________‘ΣΥΊΉνΩ…Ρή…ζ≥…ΨΏ”–¥ΏΜ·–‘÷ ΒΡ―θΜ·ΈοΓΘ
II.QΓΔRΓΔXΓΔYΓΔZΈε÷÷‘ΣΥΊΒΡ‘≠Ή”–ρ ΐ“ά¥ΈΒί‘ωΘ§≥ΐZ“‘ΆβΘ§Τδ”ύΒΡΨυΈΣΕΧ÷ήΤΎ÷ςΉε‘ΣΥΊΓΘ“―÷ΣΘΚ
ΔΌQ‘≠Ή”2pΡήΦΕ…œ”–“ΜΗωΩ’ΙλΒάΘΜ
ΔΎR‘≠Ή”ΚΥΆβL≤ψΒγΉ” ΐΈΣΤφ ΐΘΜ
ΔέX‘≠Ή”2pΙλΒά…œ÷Μ”–“ΜΕ‘Ή‘–ΐœύΖ¥ΒΡΒγΉ”ΘΜ
ΔήY‘≠Ή”ΦέΒγΉ”Θ®ΆβΈßΒγΉ”Θ©≈≈≤ΦmsnmpnΘΜ
ΔίZ‘≠Ή”M≤ψΥυ”–ΙλΒά»Ϊ≤Ω≥δ¬ζΘ§N≤ψΈό≥…Ε‘ΒγΉ”Θ§÷Μ”–1ΗωΈ¥≥…Ε‘ΒγΉ”ΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®5Θ©Z2ΘΪΒΡΚΥΆβΒγΉ”≈≈≤Φ Ϋ «________Θ§X‘ΣΥΊΜυΧ§‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤ΦΆΦ «____________ΓΘ
Θ®6Θ©Q”κYΖ÷±π–Έ≥…ΒΡΉνΦρΒΞΤχΧ§«βΜ·Έο÷–Θ§Έ»Ε®–‘Ηϋ«ΩΒΡ «________Θ®ΧνΜ·―ß ΫΘ©ΓΘ
Θ®7Θ©QΓΔRΓΔXΓΔY»ΐ÷÷‘ΣΥΊΒΡΒΎ“ΜΒγάκΡή ΐ÷Β”…¥σΒΫ–ΓΒΡΥ≥–ρΈΣ________Θ®”Ο‘ΣΥΊΖϊΚ≈Ής¥πΘ©ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥«ΩΥα–‘»ή“ΚX÷–Ω…ΡήΚ§”–Fe2+ΓΔAl3+ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() ΓΔClΘ≠÷–ΒΡ»τΗ…÷÷Θ§œ÷»ΓX»ή“ΚΫχ––Ν§–χ Β―ιΘ§ Β―ιΙΐ≥ΧΦΑ≤ζΈο»γΆΦΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
ΓΔClΘ≠÷–ΒΡ»τΗ…÷÷Θ§œ÷»ΓX»ή“ΚΫχ––Ν§–χ Β―ιΘ§ Β―ιΙΐ≥ΧΦΑ≤ζΈο»γΆΦΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©

A.ΤχΧεA «NO2
B.X»ή“Κ÷–ΩœΕ®¥φ‘ΎFe2+ΓΔAl3+ΓΔ![]() ΓΔ
ΓΔ![]()
C.»ή“ΚEΚΆΤχΧεF≤ΜΡήΖΔ…ζΜ·―ßΖ¥”Π
D.X»ή“Κ÷–≤ΜΡή»ΖΕ®ΒΡάκΉ” «Al3+ΚΆClΘ≠
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœρΫωΚ§Fe2ΘΪΓΔIΘ≠ΓΔBrΘ≠ΒΡ»ή“Κ÷–Ά®»κ ΝΩ¬»ΤχΘ§»ή“Κ÷–’β»ΐ÷÷άκΉ”ΒΡΈο÷ ΒΡΝΩΥφœϊΚΡ¬»ΤχΈο÷ ΒΡΝΩΒΡ±δΜ·»γœ¬ΆΦΥυ ΨΓΘœ¬Ν–ΥΒΖ®÷–’ΐ»ΖΒΡ «(ΓΓΓΓ)

A.œΏΕΈΔσ¥ζ±μFe2ΘΪΒΡ±δΜ·«ιΩω
B.œΏΕΈΔώ¥ζ±μBrΘ≠ΒΡ±δΜ·«ιΩω
C.a÷ΒΒ»”Ύ6
D.‘≠ΜλΚœ»ή“Κ÷–n(FeBr2)ΘΫ4mol
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ÷”–Ϋπ τΒΞ÷ AΓΔBΓΔCΚΆΤχΧεΦΉΓΔ““ΓΔ±ϊ“‘ΦΑΈο÷ DΓΔEΓΔFΓΔGΓΔHΘ§ΥϋΟ«÷°ΦδΒΡœύΜΞΉΣΜ·ΙΊœΒ»γΆΦΥυ Ψ![]() ΆΦ÷–”––©Ζ¥”ΠΒΡ…ζ≥…ΈοΚΆΖ¥”ΠΒΡΧθΦΰΟΜ”–±ξ≥ω
ΆΦ÷–”––©Ζ¥”ΠΒΡ…ζ≥…ΈοΚΆΖ¥”ΠΒΡΧθΦΰΟΜ”–±ξ≥ω![]() ΓΘ
ΓΘ
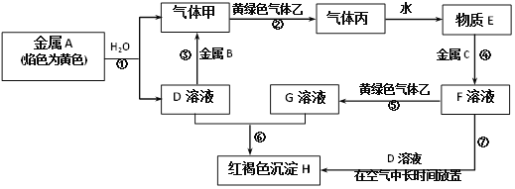
«κΗυΨί“‘…œ–≈œΔΆξ≥…œ¬Ν–ΗςΧβΘΚ
(1)–¥≥ωœ¬Ν–Έο÷ ΒΡΜ·―ß ΫΘΚB_______ΓΔ±ϊ__________ΓΘ
(2)–¥≥ωΜΤ¬Χ…ΪΤχΧε““ΒΡ“Μ÷÷”ΟΆΨ___________Θ§Ζ¥”ΠΙΐ≥ΧΔΏΩ…ΡήΙέ≤λΒΫΒΡ Β―ιœ÷œσ «______ΓΘΕ‘”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ «_______ΓΘ
(3)Ζ¥”ΠΔέ÷–ΒΡάκΉ”ΖΫ≥Χ Ϋ «_________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩAΓΔBΓΔCΓΔDΓΔEΓΔX «÷–―ß≥ΘΦϊΒΡΈόΜζΈοΘ§¥φ‘Ύ»γœ¬ΆΦΉΣΜ·ΙΊœΒΘ®≤ΩΖ÷…ζ≥…ΈοΚΆΖ¥”ΠΧθΦ଑»ΞΘ©ΓΘ
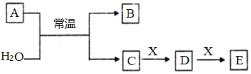
Θ®1Θ©»τAΈΣ≥ΘΦϊΒΡΫπ τΒΞ÷ Θ§«“Τδ―φ…ΪΖ¥”Π≥ ΜΤ…ΪΘ§XΡή ΙΤΖΚλ»ή“ΚΆ …ΪΘ§–¥≥ωCΚΆEΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΘΚ___________________________________________ΓΘ
Θ®2Θ©»τAΈΣΕΧ÷ήΤΎ‘ΣΥΊΉι≥…ΒΡΒΞ÷ Θ§ΗΟ‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΒΡΥ°Μ·ΈοΥα–‘Ήν«ΩΘ§‘ρXΩ…ΡήΈΣ__________Θ®ΧνΉ÷ΡΗΘ©ΓΘ
a. NaHCO3 b. Na2CO3 c.Al(OH)3 d.NaAlO2
Θ®3Θ©»τAΈΣΒ≠ΜΤ…ΪΖέΡ©Θ§‘ρAΒΡΒγΉ” ΫΈΣ_______ ΓΘ»τXΈΣ“Μ÷÷Ήν≥ΘΦϊΒΡ‘λ≥…Έ¬ “–ß”ΠΒΡΤχΧεΓΘ‘ρΦχ±πΒ»≈®Ε»ΒΡDΓΔEΝΫ÷÷»ή“ΚΘ§Ω…―Γ‘ώΒΡ ‘ΦΝΈΣ___ΓΘΘ®ΧνΉ÷ΡΗΘ©
a.―ΈΥα b.CaCl2»ή“Κ c.Α±Υ° d.≥Έ«ε ·Μ“Υ°
Θ®4Θ©»τAΈΣ―θΜ·ΈοΘ§X «FeΘ§»ή“ΚD÷–Φ”»κKSCN»ή“Κ±δΚλΓΘ‘ρA”κΥ°Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ÷–―θΜ·ΦΝ”κΜΙ‘≠ΦΝΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ___________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ÷–Ιζ¥ΪΆ≥ΈΡΜ·÷–Αϋά®–μΕύΩΤΦΦ÷Σ ΕΓΘœ¬Ν–Ι≈”ο÷–≤Μ…φΦΑΜ·―ß±δΜ·ΒΡ «
«ßΧ‘ΆρδθΥδ–ΝΩύΘ§¥ΒΨΓΩώ…≥ ΦΒΫΫπ |
ΑΨΒ®Ζ·(CuSO4ΓΛ5H2O) ΧζΗΣΘ§ΨΟ÷°“ύΜ·ΈΣΆ≠ |
Ζ≤ ·Μ“(CaCO3)Θ§ Ψ≠ΜπΖΌΝΕΈΣ”Ο |
ΒΛ…Α(HgS)…’÷°≥…Υ°“χΘ§Μΐ±δ”÷≥…ΒΛ…Α |
A | B | C | D |
A. A B. B C. C D. D
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com