
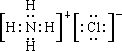 ������DΪKCl��
������DΪKCl��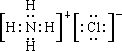 ��
��
| ||
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��c��H+��=0.1mol/L�ļ�����Һ�У�HCOO-��H+ ��Ŀ֮��Ϊ0.1 NA |
| B��ͨ������£�����Һ̬�����ǹ�̬������I-I����Br-Br�� |
| C����ϡ��ˮ��μ���ϡ�����У�����ҺpH=7ʱ��2C��NH4+��=C��SO42-�� |
D���ϳ�˳����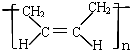 ���ĵ�����CH2=CH-CH=CH2 ���ĵ�����CH2=CH-CH=CH2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

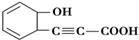 ������HBr����1��1�ӳɵIJ��������
������HBr����1��1�ӳɵIJ���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ӧ��ȡNaOH������/g | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
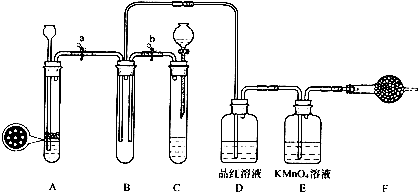
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
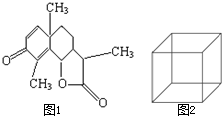 ��1��ͼ1��һ�����׳�ҩ--ɽ����Ľṹ��ʽ����ȷ�������ʽΪ��
��1��ͼ1��һ�����׳�ҩ--ɽ����Ľṹ��ʽ����ȷ�������ʽΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ѧҩƷ�Ż𣬶�Ҫ������ˮ����ĭ�������� |
| B���������ƹ�����Դ��ʵ�ֵ�̼�����;��֮һ |
| C��ʳƷ��װ���г�����С������ʯ�ң�Ŀ���Ƿ�ֹʳƷ�������� |
| D����ά���������ڿ�ˮ��Ϊ�����ǣ��ʿ��������Ӫ������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com