

±ķ1 ĪļÖŹ³ĮµķŹ±µÄpH
Īļ ÖŹ | Fe£ØOH£©3 | Fe£ØOH£©2 | Cu£ØOH£©2 |
æŖŹ¼³ĮµķŹ±µÄpH | 2.7 | 7.6 | 5.2 |
ĶźČ«³ĮµķµÄpH | 3.7 | 9.6 | 6.4 |
±ķ2 ±øŃ”ŹŌ¼Į
Šņ ŗÅ | A | B | C | D | E |
»ÆѧŹ½ | NaOH | CuO | H2O2 | Ļ”HNO3 | KMnO4 |
»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©X_________£»Y_________”££ØĢīŠ“±øŃ”ŹŌ¼ĮÖŠµÄŠņŗÅ£©
£Ø2£©³Įµķ£Ø¢ņ£©µÄ»ÆѧŹ½_____£¬Ö¤Ć÷²½Öč¢ŪÖŠ³ĮµķŹĒ·ńĶźČ«µÄ²Ł×÷·½·ØŹĒ____________”£
£Ø3£©µŚ¢Ż²½Öč²Ł×÷ÖŠ£¬ĖłŠč×°ÖĆČēĶ¼ĖłŹ¾£ŗ

°“ĘųĮ÷“Ó×óÖĮÓŅµÄ·½Ļņ£¬ø÷×°ÖĆ½ÓæŚµÄĮ¬½ÓĖ³ŠņĪŖ_________”£
D×°ÖƵÄ×÷ÓĆŹĒ______________________£»ĄķÓÉ______________________”£
½āĪö£ŗ“ÖCuOÖŠÓŠFeOŗĶ²»ČÜÓŚĖįµÄŌÓÖŹ£¬¢Ł³żČ„²»ČÜŠŌŌÓÖŹ£¬¢ńČÜŅŗĪŖCuCl2”¢FeCl2ČÜŅŗ”£¢Ś¢Ū³żFe2£«“Ó±øŃ”ŹŌ¼ĮŗĶpHæ“£¬¢ņĪŖFe£ØOH£©3£¬¢ŚŌņŅŖ½«Fe2£«Ńõ»ÆĪŖFe3£«£¬ÓĆH2O2²»ŅżČėŌÓÖŹ£¬ÓĆHNO3ŗĶKMnO4»įŅżČėŌÓÖŹ£¬¹ŹXĪŖH2O2”£Ź¹Fe3£«³ĮµķµÄŹŌ¼ĮÓŠNaOHŗĶCuO£¬NaOH»įŅżČėŌÓÖŹĒŅ²»Ņ×æŲÖĘpH£¬¶ųCuOÄÜÓėH£«½įŗĻ£¬Ź¹Fe3£«Ė®½ā½ųŠŠµ½µ×£¬³żČ„Fe3£«£¬ĒŅ²»ŅżČėŌÓÖŹ£¬¹ŹYĪŖCuO”£Ö¤Ć÷Fe3£«³ĮµķĶźČ«£¬¼ģŃéĀĖŅŗŹĒ·ń“ęŌŚFe3£«¼“æÉ”£ÓÉÓŚCuCl2Ņ×Ė®½ā£¬ĖłŅŌCuCl2”¤2H2O”śCuCl2ŅŖŌŚHClĘų·ÕÖŠ½ųŠŠ£¬ŅĒĘ÷Į¬½Ó°“£ŗÖĘĘų”Ŗ”ŖøÉŌļ”Ŗ”Ŗ·“Ó¦”Ŗ”Ŗ·ĄæÕĘųÖŠĖ®Ęū½ųČėµÄĖ³Šņ×é×°”£
“š°ø£ŗ£Ø1£©C B
£Ø2£©Fe£ØOH£©3 ȔɣĮæĀĖŅŗÓŚŹŌ¹ÜÖŠ£¬µĪČėKSCN£¬ČōĀĖŅŗ²»±äŗģ£¬ĖµĆ÷ĘäÖŠĪŽFe3£«£¬³ĮµķŅŃĶźČ«£ØĘäĖū·½·ØŗĻĄķ¾łæÉ£©
£Ø3£©gabcdfe D×°ÖĆÖŠÉś³ÉµÄHClŌŚBÖŠĀČ»ÆĶŹÜČČĶŃ½į¾§Ė®Ź±£¬æÉ·ĄÖ¹ĀČ»ÆĶĖ®½ā ĶØČėHCl£¬c£ØH£«£©Ōö“ó£¬Ź¹·“Ó¦Cu2£«£«2H2O![]() Cu£ØOH£©2£«2H£«Ę½ŗāĻņ×óŅĘ¶Æ£¬
Cu£ØOH£©2£«2H£«Ę½ŗāĻņ×óŅĘ¶Æ£¬
ŅÖÖĘĖ®½ā·¢Éś£¬ÄܵƵ½½Ļ¶ąµÄĪŽĖ®ĀČ»ÆĶ


 ¾ŁŅ»·“ȿʌĩ°Ł·Ö³å“Ģ¾ķĻµĮŠ“š°ø
¾ŁŅ»·“ȿʌĩ°Ł·Ö³å“Ģ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ÆѧŠĖȤŠ”×éÄāÓĆ“ÖŃõ»ÆĶ(ŗ¬ÉŁĮæĶ·Ū”¢Ńõ»ÆĢś¼°²»ČÜÓŚĖįµÄŌÓÖŹ)ÖĘČ”
ĪŽĖ®ĀČ»ÆĶ£¬ĘäÖʱø²½ÖčČēĻĀ£ŗ
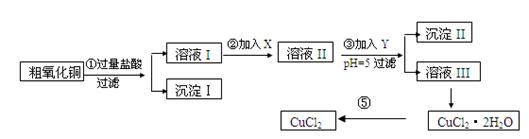
ŹµŃé¹ż³ĢÖŠĖłÓƵÄĪļÖŹX£¬Y¼°pHæŲÖĘ²ĪÕÕĻĀ±ķČ·¶Ø£ŗ
±ķI
| ĪļÖŹ | æŖŹ¼³ĮµķŹ±pH | ĶźČ«³ĮµķŹ±pH |
| Fe(OH)3 | 2£®7 | 3£®7 |
| Fe(OH)2 | 7£®6 | 9£®6 |
| Cu(OH)2 | 5£®2 | 6£®4 |
±ķ¢ņ
| Ńõ»Æ¼Į | µ÷½ŚpHµÄĪļÖŹ | ||
| A | Ė«ŃõĖ® | D | °±Ė® |
| B | øßĆĢĖį¼Ų | E | ¼īŹ½Ģ¼ĖįĶ |
| C | ĀČĖ® | F | Ńõ»ÆĶ |
ĒėĢīŠ“ĻĀĮŠæÕ°×
(1)³Įµķ¢ņµÄ³É·Ö(»ÆѧŹ½)ŹĒ ”£
(2)²½Öč¢Ś¼ÓČėµÄŹŌ¼ĮXæÉŃ”ÓƱķ¢ņÖŠµÄ (ĢīŠņŗÅ)£¬Ęä×÷ÓĆŹĒ ”£
(3)²½Öč¢Ū¼ÓČėµÄŹŌ¼ĮYæÉŃ”ÓƱķ¢ņÖŠµÄ (ĢīŠņŗÅ)£¬æŲÖĘpH£½5µÄÄæµÄŹĒ ”£
(4)²½Öč¢ŻŅŖµĆµ½ĪŽĖ®CuCl2£¬Ó¦æŲÖʵÄĢõ¼žŹĒ ”£
(5)²½Öč¢ŁÖŠĖł·¢ÉśµÄČ«²æ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ÆѧŠĖȤŠ”×éÄāÓĆ“ÖŃõ»ÆĶ(ŗ¬ÉŁĮæĶ·Ū”¢Ńõ»ÆĢś¼°²»ČÜÓŚĖįµÄŌÓÖŹ)ÖĘČ”
ĪŽĖ®ĀČ»ÆĶ£¬ĘäÖʱø²½ÖčČēĻĀ£ŗ
ŹµŃé¹ż³ĢÖŠĖłÓƵÄĪļÖŹX£¬Y¼°pHæŲÖĘ²ĪÕÕĻĀ±ķČ·¶Ø£ŗ
±ķI
| ĪļÖŹ | æŖŹ¼³ĮµķŹ±pH | ĶźČ«³ĮµķŹ±pH |
| Fe(OH)3 | 2£®7 | 3£®7 |
| Fe(OH)2 | 7£®6 | 9£®6 |
| Cu(OH)2 | 5£®2 | 6£®4 |
±ķ¢ņ
| Ńõ»Æ¼Į | µ÷½ŚpHµÄĪļÖŹ | ||
| A | Ė«ŃõĖ® | D | °±Ė® |
| B | øßĆĢĖį¼Ų | E | ¼īŹ½Ģ¼ĖįĶ |
| C | ĀČĖ® | F | Ńõ»ÆĶ |
ĒėĢīŠ“ĻĀĮŠæÕ°×
(1)³Įµķ¢ņµÄ³É·Ö(»ÆѧŹ½)ŹĒ ”£
(2)²½Öč¢Ś¼ÓČėµÄŹŌ¼ĮXæÉŃ”ÓƱķ¢ņÖŠµÄ (ĢīŠņŗÅ)£¬Ęä×÷ÓĆŹĒ ”£
(3)²½Öč¢Ū¼ÓČėµÄŹŌ¼ĮYæÉŃ”ÓƱķ¢ņÖŠµÄ (ĢīŠņŗÅ)£¬æŲÖĘpH£½5µÄÄæµÄŹĒ ”£
(4)²½Öč¢ŻŅŖµĆµ½ĪŽĖ®CuCl2£¬Ó¦æŲÖʵÄĢõ¼žŹĒ ”£
(5)²½Öč¢ŁÖŠĖł·¢ÉśµÄČ«²æ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗÓ±±Ź”»Ęęč֊ѧø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ij»ÆѧŠĖȤŠ”×éÄāÓĆ“ÖŃõ»ÆĶ(ŗ¬ÉŁĮæĶ·Ū”¢Ńõ»ÆĢś¼°²»ČÜÓŚĖįµÄŌÓÖŹ)ÖĘČ”
ĪŽĖ®ĀČ»ÆĶ£¬ĘäÖʱø²½ÖčČēĻĀ£ŗ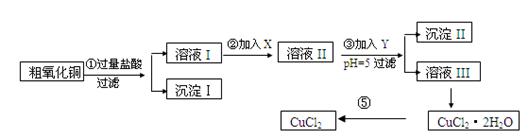
ŹµŃé¹ż³ĢÖŠĖłÓƵÄĪļÖŹX£¬Y¼°pHæŲÖĘ²ĪÕÕĻĀ±ķČ·¶Ø£ŗ
±ķI
| ĪļÖŹ | æŖŹ¼³ĮµķŹ±pH | ĶźČ«³ĮµķŹ±pH |
| Fe(OH)3 | 2£®7 | 3£®7 |
| Fe(OH)2 | 7£®6 | 9£®6 |
| Cu(OH)2 | 5£®2 | 6£®4 |
| Ńõ»Æ¼Į | µ÷½ŚpHµÄĪļÖŹ | ||
| A | Ė«ŃõĖ® | D | °±Ė® |
| B | øßĆĢĖį¼Ų | E | ¼īŹ½Ģ¼ĖįĶ |
| C | ĀČĖ® | F | Ńõ»ÆĶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźŗÓ±±Ź”ø߶žÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ij»ÆѧŠĖȤŠ”×éÄāÓĆ“ÖŃõ»ÆĶ(ŗ¬ÉŁĮæĶ·Ū”¢Ńõ»ÆĢś¼°²»ČÜÓŚĖįµÄŌÓÖŹ)ÖĘČ”
ĪŽĖ®ĀČ»ÆĶ£¬ĘäÖʱø²½ÖčČēĻĀ£ŗ
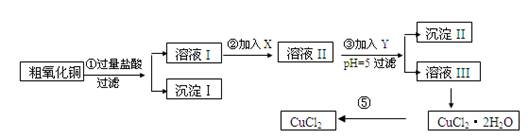
ŹµŃé¹ż³ĢÖŠĖłÓƵÄĪļÖŹX£¬Y¼°pHæŲÖĘ²ĪÕÕĻĀ±ķČ·¶Ø£ŗ
±ķI
|
ĪļÖŹ |
æŖŹ¼³ĮµķŹ±pH |
ĶźČ«³ĮµķŹ±pH[Ą“Ō“:Zxxk.Com] |
|
Fe(OH)3 |
2£®7 |
3£®7 |
|
Fe(OH)2 |
7£®6 |
9£®6 |
|
Cu(OH)2 |
5£®2 |
6£®4 |
±ķ¢ņ
|
Ńõ»Æ¼Į |
µ÷½ŚpHµÄĪļÖŹ |
||
|
A |
Ė«ŃõĖ® |
D |
°±Ė® |
|
B |
øßĆĢĖį¼Ų |
E |
¼īŹ½Ģ¼ĖįĶ |
|
C |
ĀČĖ® |
F |
Ńõ»ÆĶ |
ĒėĢīŠ“ĻĀĮŠæÕ°×
(1)³Įµķ¢ņµÄ³É·Ö(»ÆѧŹ½)ŹĒ ”£
(2)²½Öč¢Ś¼ÓČėµÄŹŌ¼ĮXæÉŃ”ÓƱķ¢ņÖŠµÄ (ĢīŠņŗÅ)£¬Ęä×÷ÓĆŹĒ ”£
(3)²½Öč¢Ū¼ÓČėµÄŹŌ¼ĮYæÉŃ”ÓƱķ¢ņÖŠµÄ (ĢīŠņŗÅ)£¬æŲÖĘpH£½5µÄÄæµÄŹĒ ”£
(4)²½Öč¢ŻŅŖµĆµ½ĪŽĖ®CuCl2£¬Ó¦æŲÖʵÄĢõ¼žŹĒ ”£
(5)²½Öč¢ŁÖŠĖł·¢ÉśµÄČ«²æ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com