
(10��)�ס��ҡ�����������������ˮ�����ʣ��ֱ���NH4+��Ba2+��Mg2+��H+��OH����Cl����HCO3����SO42���еIJ�ͬ�����Ӻ������Ӹ�һ����ɡ���֪��
�ٽ�����Һ�ֱ��������������ʵ���Һ��ϣ����а�ɫ�������ɣ�
��0.1mol/L����Һ��c(H+)>0.1mol/L��
�������Һ�е���AgNO3��Һ�в�����ϡHNO3�İ�ɫ�������ɡ��ش�
��1������Һ��
��2������Һ�ĵ��뷽��ʽ
��3������ҺPH 7, ԭ���÷���ʽ��ʾ
��4���ҺͶ���Ӧ�����ӷ���ʽ
��1��NH4HCO3 ��2��H2SO4===2H+ +SO42�� ��3�� �� Mg2++2H2O === Mg(OH)2 +2H+
��4��H+ + HCO3��=== CO2��+H2O
��������
������������ݢ��е���Ϣ��֪���Ƕ�Ԫ�ᣬ������H2SO4�����ݢ�����������֪���к���Cl-���ٽ�Ϣ����ṩ��Ϣ�����������������ʻ�Ͼ�������ɫ����������Ƴ�����Ba��OH��2������H2SO4������MgCl2������NH4HCO3��(1) ����Һ��NH4HCO3��(2) ������ǿ����ʣ�����뷽��ʽ�ǣ�H2SO4===2H+ +SO42����(3) ����ҺPH��7, ԭ����������þ���������Һ�п���ˮ�⣬�����ӷ���ʽ��ʾΪ��Mg2++2H2O === Mg(OH)2 +2H+��(4) �ҺͶ���Ӧ�����ӷ���ʽΪ��H++HCO3��===CO2��+H2O��
���㣺�������ӵļ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ֽ���������ĩ15 g�������������ᷴӦʱ���ɱ�״����11.2 L������������������Ľ����������
A��Zn��Fe B��Zn��Ag C��Al��Cu D��Mg��Al
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ˫Ѽɽ�и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A������ԭ��ʧ����Խ�࣬�仹ԭ��Խǿ
B��CH4��Ħ������Ϊ16g
C��10 mL��������Ϊ98%��H2SO4����ˮϡ����100 mL��H2SO4����������Ϊ9.8%
D����������ʱ������ܵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���ຣʡ�����и�����ѧ�ڵ�һ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
��15�֣�Ϊ��̽��H2O2��H2SO3��Br2�����Ե����ǿ�����������ʵ�飨�г���������ȥ������ش��������⣺

��1������A������_________����������___________��
��2��������B�μ�Һ�岢����Ҫ������c��ԭ����____________________________��
��3��ʵ���¼���£��벹ȫ�հף���
���� | ʵ����� | ʵ������ | ʵ����� |
�� | ����a����μ���H2SO3��Һ������ | ________________ | __________________________ |
�� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | __________________________ |
��4��������У���ʼʱ��ɫ�����Ա仯��ԭ���ǣ�д��һ����_______________________��
������з�Ӧ�����ӷ���ʽ_________________________________________________��
���������Ҫ��Ӧ�����ӷ���ʽ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���ຣʡ�����и�����ѧ�ڵ�һ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����
A������ȼ��ʱ��ȫ���Ļ�ѧ��ת��Ϊ����
B����AgCl��Һ�м������ᣬKsp���
C�������ڼ�������R2-����Rһ������VIA��
D�������������ٵĽ���Ԫ�أ�һ����������������Ľ���Ԫ�ػ�����ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�������ɽ�ظ�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��a LAl2(SO4)3��(NH4)2SO4�Ļ������Һ�м���b molBaCl2��ǡ��ʹ��Һ�е�SO42��������ȫ���������������ǿ����ȿɵõ�c molNH3������ԭ��Һ�е�Al3+����Ũ��(mol/L)Ϊ
A��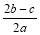 B��
B��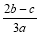 C��
C�� 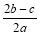 D��
D��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�������ɽ�ظ�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���з�Ӧ�У��������뻹ԭ�����ʵ����Ĺ�ϵΪ1��2����
A��4HCl+MnO2===MnCl2+Cl2��+2H2O
B��I2+2NaClO3===2NaIO3+Cl2
C��2CH3COOH+Ca(ClO)2===2HClO+Ca(CH3COO)2
D��Fe + 2H+===2Fe2+ + H2 ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���20��00 mL 1��000 mol��L��1��ˮ�е���1��000 mol��L��1���ᣬ��ҺpH���¶��������������仯������ͼ��ʾ�������й�˵���������

A��a��d�������Һ��ˮ�����ӻ�Kw(a)>Kw(d)
B�����˰�ˮϡ�ͣ���Һ�ĵ�����������
C��c��ʱ�����������V(HCl)<20 mL
D�������£�a��İ�ˮ���볣��Ϊ mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A.���ʵ���Ũ�Ⱥ��������ͬ������ʹ�����Һ����������п��Ӧʱ����ʼʱ���߲���H2���ʻ������
B.100 mL 1 mol��L-1�������50 mL 2 mol��L-1�����ᣬ�ֱ���������п��Ӧʱ�����߷ų�H2���ʺ����������
C.100 mL pH=3��H2SO4��HCl��Һ��������п��Ӧ�ų�H2���������
D.100 mL pH=3�������������Һ��������п��Ӧ������H2���������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com