
(15Зж) ФГЭЌбЇЩшМЦСЫШчЯТЭМЫљЪОзАжУЃЈВПЗжМаГжзАжУвбТдШЅЃЉЃЌИУзАжУПЩвдгУРДНјааЖрЯю
ЪЕбщбаОПЁЃ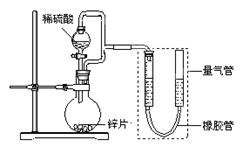
ЧыЛиД№ЃК
ЃЈ1ЃЉгУЩЯЪізАжУЬНОПгАЯьЛЏбЇЗДгІЫйТЪЕФвђЫиЁЃ
ЂйдВЕзЩеЦПжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЪЧ ЁЃ
ЂкгУЩЯЪізАжУНјааЪЕбщЃЌвдЩњГЩ9.0 mLЦјЬхЮЊМЦЪБжеЕуЃЌНсЙћЮЊt1ЃОt2ЁЃ
| ађКХ | V(H2SO4)/mL | c(H2SO4)/molЁЄLЃ1 | t/s |
| Ђё | 40 | 1 | t1 |
| Ђђ | 40 | 4 | t2 |
ЃЈ15ЗжЃЉЃЈ1ЃЉЂйZn+2H+ ЃНZn2++H2ЁќЃЈ1ЗжЃЉ
ЂкдкЦфЫќЬѕМўвЛЖЈЪБЃЌЛЏбЇЗДгІЫйТЪЫцЗДгІЮяХЈЖШЕФдіДѓЖјдіДѓЃЈ2ЗжЃЉЂлabcЃЈ2ЗжЃЉ
ЃЈ2ЃЉЂй ЁС100%ЃЈ2ЗжЃЉ
ЁС100%ЃЈ2ЗжЃЉ
ЂкД§ЦјЬхРфШДКѓЃЌЕїећгвБпСПЦјЙмИпЖШЃЌЪЙЦфзѓгвЙмжаЫЎУцЯрЦНЃЈ2ЗжЃЉ ЂлЮогАЯьЃЈ2ЗжЃЉ
ЃЈ3ЃЉЂйc ЃЈ2ЗжЃЉ ЂкСПЦјЙмзѓЙмЕФЫЎУцЩЯЩ§ЃЌгвЙмЕФЫЎУцЯТНЕЃЈ2ЗжЃЉ
НтЮіЪдЬтЗжЮіЃКЃЈ1ЃЉЂйаПКЭЯЁСђЫсЗДгІЕФРызгЗНГЬЪНЮЊZn+2H+ ЃНZn2++H2ЁќЁЃ
ЂкИљОнБэжаЪ§ОнПЩжЊЃЌЪЕбщЂёКЭЂђЯрБШЃЌЪЕбщЂђжаСђЫсЕФХЈЖШДѓЃЌгУЪБЩйЃЌетЫЕУїдкЦфЫќЬѕМўвЛЖЈЪБЃЌЛЏбЇЗДгІЫйТЪЫцЗДгІЮяХЈЖШЕФдіДѓЖјдіДѓЁЃ
ЂлЫљВтЕУЕФЗДгІЫйТЪОљДѓгкЩЯЪіЪЕбщЖдгІЕФЪ§ОнЃЌетЫЕУїДжаПЦЌжаЫљКЌдгжЪгыаПЙЙГЩСЫдЕчГиЃЌЧвдгжЪЕФН№ЪєадШѕгкаПЕФЃЌЫљвдЗћКЯЬѕМўЕФЪЧЪЏФЋЁЂвјКЭЭЃЌД№АИбЁabcЁЃ
ЃЈ2ЃЉЂйЧтЦјЕФЬхЛ§дкБъзМзДПіЯТЪЧVLЃЌдђЧтЦјЕФЮяжЪЕФСПЪЧ molЃЌдђИљОнЗНГЬЪНПЩжЊЃЌВЮМгЗДгІЕФаПЕФЮяжЪЕФСПЪЧ
molЃЌдђИљОнЗНГЬЪНПЩжЊЃЌВЮМгЗДгІЕФаПЕФЮяжЪЕФСПЪЧ molЃЌЦфжЪСПЪЧ
molЃЌЦфжЪСПЪЧ molЁС65g/molЃН
molЁС65g/molЃН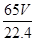 gЃЌЫљвдДжаПЦЌЕФДПЖШЮЊ
gЃЌЫљвдДжаПЦЌЕФДПЖШЮЊ ЁС100%ЁЃ
ЁС100%ЁЃ
ЂкгЩгкЦјЬхЕФЬхЛ§ЪмЮТЖШКЭбЙЧПгАЯьДѓЃЌЫљвддкЖСЪ§ЧАЖдСПЦјЙмЕФВйзїЪЧД§ЦјЬхРфШДКѓЃЌЕїећгвБпСПЦјЙмИпЖШЃЌЪЙЦфзѓгвЙмжаЫЎУцЯрЦНЁЃ
ЂлгЩгкзАжУЪЧЗтБеЕФЃЌЫљвдЯЁСђЫсЕФЬхЛ§ВЂВЛФмгАЯьНјШыСПЦјЙмЕФЧтЦјЬхЛ§ЃЌвђДЫЖдЪЕбщНсЙћЮогАЯьЁЃ
ЃЈ3ЃЉЂйвЊгУЩЯЪізАжУбщжЄЩњЬњдкГБЪЊПеЦјжаЛсЗЂЩњЮќбѕИЏЪДЃЌдђЕчНтжЪШмвКЕФЫсадгІИУКмШѕЃЌЩѕжСЯджаадЛђМюадЁЃТШЛЏяЇШмгкЫЎЯдЫсадЃЌЯЁСђЫсШмгкЫЎЯдЫсадЃЌввДМЪЧЗЧЕчНтжЪЃЌЬМЫсФЦШмвКЯдМюадЃЌЫљвдД№АИбЁcЁЃ
ЂкШчЙћЗЂЩњЮќбѕИЏЪДЃЌдђзАжУжабЙЧПНЕЕЭЃЌЫљвдФмжЄУїЩњЬњдкГБЪЊПеЦјжаЛсЗЂЩњЮќбѕИЏЪДЕФЯжЯѓЪЧСПЦјЙмзѓЙмЕФЫЎУцЩЯЩ§ЃЌгвЙмЕФЫЎУцЯТНЕЁЃ
ПМЕуЃКПМВщЭтНчЬѕМўЖдЗДгІЫйТЪЕФгАЯьЁЂДПЖШВтЖЈЁЂЦјЬхЬхЛ§ВтЖЈЁЂН№ЪєЕФЮќбѕИЏЪДвдМАЪЕбщЗНАИЩшМЦгыЦРМлЕШ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКМЦЫуЬт
ЙЄвЕЩЯвдАБЦјЮЊдСЯ(ВЌююКЯН№ЭјЮЊДпЛЏМС)ДпЛЏбѕЛЏЗЈжЦЯѕЫсЕФЙ§ГЬШчЯТЃК
|

 ЃЈ1ЃЉМКжЊЗДгІвЛОЗЂЩњЃЌВЌююКЯН№ЭјОЭЛсДІгкКьШШзДЬЌЁЃаДГіАБДпЛЏбѕЛЏЕФЛЏбЇЗНГЬЪНЃК______________________________________________ЃЛЕБЮТЖШЩ§ИпЪБЃЌИУЗДгІЕФЦНКтГЃЪ§KжЕ___________(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЁЃ
ЃЈ1ЃЉМКжЊЗДгІвЛОЗЂЩњЃЌВЌююКЯН№ЭјОЭЛсДІгкКьШШзДЬЌЁЃаДГіАБДпЛЏбѕЛЏЕФЛЏбЇЗНГЬЪНЃК______________________________________________ЃЛЕБЮТЖШЩ§ИпЪБЃЌИУЗДгІЕФЦНКтГЃЪ§KжЕ___________(ЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЁЃ 2NH3ЃЌИУЗДгІдкЙЬЖЈШнЛ§ЕФУмБеШнЦїжаНјааЁЃЯТСаИїЯюБъжОзХИУЗДгІДяЕНЛЏбЇЦНКтзДЬЌЕФЪЧ____________(ЬюађКХ)
2NH3ЃЌИУЗДгІдкЙЬЖЈШнЛ§ЕФУмБеШнЦїжаНјааЁЃЯТСаИїЯюБъжОзХИУЗДгІДяЕНЛЏбЇЦНКтзДЬЌЕФЪЧ____________(ЬюађКХ)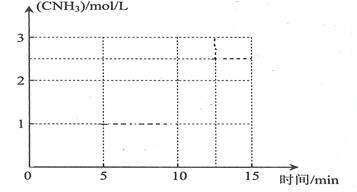
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЃЈ14Зж УППе2ЗжЃЉЛЏЙЄдСЯКьЗЏФЦ(жиИѕЫсФЦ:Na2Cr2O7ЁЄ2H2O)жївЊЪЧвдИѕЬњПѓ(жївЊГЩЗжЮЊFeOЁЄCr2O3,ЛЙКЌгаAl2O3ЁЂSiO2ЕШдгжЪ)ЮЊжївЊдСЯЩњВњ,ЦфжївЊЙЄвеСїГЬШчЯТ:
ВНжшЂйжажївЊЗДгІЕФЛЏбЇЗНГЬЪНШчЯТ:
4FeOЁЄCr2O3+8Na2CO3+7O2 8Na2CrO4+2Fe2O3+8CO2
8Na2CrO4+2Fe2O3+8CO2
ЃЈ1ЃЉ ЂйжаЗДгІЪЧдкЛизЊвЄжаНјааЕФ,ЗДгІЪБашВЛЖЯНСАш,ЦфзїгУЪЧЁЁ ЁЃ
ЃЈ2ЃЉ дгжЪAl2O3дкЂйжазЊЛЏЕФЛЏбЇЗДгІЗНГЬЪНЮЊЁЁЁЁЁЁ ЁЁЁЃ
ЃЈ3ЃЉ гУЛЏбЇЦНКтвЦЖЏдРэЫЕУїЂлжажѓЗаЕФзїгУЪЧЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁ(гУРызгЗНГЬЪННсКЯЮФзжЫЕУї),ШєЕїНкpHЙ§ЕЭВњЩњЕФгАЯьЪЧЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЁЃ
ЃЈ4ЃЉ ЂнжаЫсЛЏЪЧЪЙCrO42-зЊЛЏЮЊCr2O72-аДГіИУЗДгІЕФРызгЗНГЬЪН:ЁЁ ЁЃ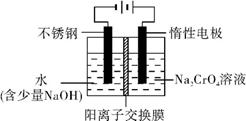
ЃЈ5ЃЉ ЙЄвЕЩЯЛЙПЩгУЕчНтЗЈжЦБИжиИѕЫсФЦ,ЦфзАжУЪОвтЭМШчЩЯЁЃ
вѕМЋЕФЕчМЋЗДгІЪНЮЊЁЁ ;
бєМЋЕФЕчМЋЗДгІЪНЮЊЁЁ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
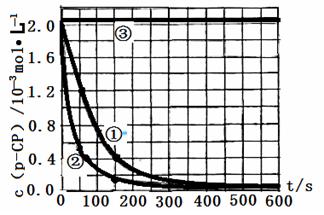
FentonЗЈГЃгУгкДІРэКЌФбНЕНтгаЛњЮяЕФЙЄвЕЗЯЫЎЃЌЭЈГЃЪЧдкЕїНкКУPHКЭFe2+ХЈЖШЕФЗЯЫЎжаМгШыH2O2ЃЌЫљВњЩњЕФєЧЛљздгЩЛљФмбѕЛЏНЕНтЮлШОЮяЁЃЯждЫгУИУЗНЗЈНЕНтгаЛњЮлШОЮяp-CPЃЌЬНОПгаЙивђЫиЖдИУНЕНтЗДгІЫйТЪЕФгАЯьЁЃ
ЁОЪЕбщЩшМЦЁППижЦp-CPЕФГѕЪМХЈЖШЯрЭЌЃЌКуЖЈЪЕбщЮТЖШдк298KЛђ313KЃЈЦфгрЪЕбщЬѕМўМћЯТБэЃЉЃЌЩшМЦШчЯТЖдБШЪдбщЁЃ
ЃЈ1ЃЉЧыЭъГЩвдЯТЪЕбщЩшМЦБэЃЈБэжаВЛвЊСєПеИёЃЉЁЃ
| ЪЕбщ БрКХ | ЪЕбщФПЕФ | T/K | pH | c/10-3molЁЄL-1 | |
| H2O2 | Fe2+ | ||||
| Ђй | ЮЊвдЯТЪЕбщзїВЮПМ | 298 | 3 | 6.0 | 0.30 |
| Ђк | ЬНОПЮТЖШЖдНЕНтЗДгІЫйТЪЕФгАЯь | | | | |
| Ђл | | 298 | 10 | 6.0 | 0.30 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЮЊСЫбаОПЭтНчЬѕМўЖдЙ§бѕЛЏЧтЗжНтЫйТЪЕФгАЯьЃЌФГЭЌбЇзіСЫвдЯТЪЕбщЃЌЧыЛиД№ЯТСаЮЪЬтЁЃ
| БрКХ | Вйзї | ЪЕбщЯжЯѓ |
| Ђй | ЗжБ№дкЪдЙмAЁЂBжаМгШы5 mL 5% H2O2ШмвКЃЌИїЕЮШы2ЕЮ1 mol/L FeCl3ШмвКЁЃД§ЪдЙмжаОљгаЪЪСПЦјХнГіЯжЪБЃЌНЋЪдЙмAЗХШыЪЂга5ЁцзѓгвРфЫЎЕФЩеБжаНўХнЃЛНЋЪдЙмBЗХШыЪЂга40ЁцзѓгвШШЫЎЕФЩеБжаНўХнЁЃ | ЪдЙмAжаВЛдйВњЩњЦјХнЃЛ ЪдЙмBжаВњЩњЕФЦјХнСПдіДѓЁЃ |
| Ђк | СэШЁСНжЇЪдЙмЗжБ№МгШы5 mL 5% H2O2ШмвККЭ5 mL 10% H2O2ШмвК | ЪдЙмAЁЂBжаОљЮДУїЯдМћЕНгаЦјХнВњЩњЁЃ |

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ЯТСаЪТЪЕ(ГЃЮТЯТ)ВЛФмЫЕУїДзЫсЪЧШѕЕчНтжЪЕФЪЧ
| AЃЎДзЫсФЦШмвКpH>7 |
| BЃЎДзЫсШмвКФмШмНтЬМЫсИЦ |
| CЃЎ0ЃЎ1molЁЄL-1ДзЫсШмвКpH=2ЃЎ9 |
| DЃЎpH=1ЕФДзЫсШмвКЯЁЪЭ100БЖКѓpH<3 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
25CЪБЃЌгУХЈЖШЮЊ0.1000 mol/LЕФNaOHШмвКЕЮЖЈ20. 00 mL 0 .1000 molЁЄL-1ЕФ
CH3COOH.ЁЃЕЮЖЈЧњЯпШчЭМЫљЪОЁЃЯТСаЗжЮіДэЮѓЕФЪЧ
| AЃЎCЕуЕФШмвКЃКcЃЈCH3COOвЛЃЉ+c(CH.3COOH)>c(Na+) |
| BЃЎBЕуЕФШмвКc (CH3COOвЛ)>c(Na+ЃЉ>c(H+ЃЉ> c(OH-) |
| CЃЎAЕуЕФзнзјБъжЕЮЊl |
| DЃЎDЕуШмвК2c(CH3COOH)ЪЎc(H+) =c(OHЁЊ)ЁЊc(CH3COOЁЊ) |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ЯТСагаЙиЮЪЬтгыбЮЕФЫЎНтЮоЙиЕФЪЧЃЈ ЃЉ
| AЃЎNH4ClгыZnCl2ШмвКПЩзїКИНгН№ЪєЪБЕФГ§атМС |
| BЃЎгУNaHCO3гыAl2(SO4)3СНжжШмвКПЩжЦГЩХнФУ№Л№МС |
| CЃЎГЃЮТЯТВтЕУNaHSO3ШмвКЕФpHаЁгк7 |
| DЃЎЪЕбщЪвЪЂЗХNa2CO3ШмвКЕФЪдМСЦПВЛФмгУФЅПкВЃСЇШћ |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
ГЃЮТЯТЃЌ0.1molЁЄLЃ1CH3COONaШмвКжаЃЌЮЂСЃХЈЖШМфЙиЯЕе§ШЗЕФЪЧ
| AЃЎc(Na+)=c(CH3COOЃ) ЃОc(OHЃ)=c(H+) |
| BЃЎc(OHЃ)=c(H+)+ c(CH3COOH) |
| CЃЎc(Na+) + c(H+)= c(CH3COOЃ) +c(OHЃ) |
| DЃЎc(CH3COOH) + c(CH3COOЃ) = c(Na+)+ c(H+) |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com