
ij��Na2O���ʵ�Na2O2�����������ʵ��ⶨ����Ʒ�Ĵ��ȡ�
�ɹ�ѡ���װ����ͼ��
�ɹ�ѡ���ҩƷ:CaCO3���壬6mol/L���ᣬ����ˮ��
��ش���������:
(1)������װ�ÿ�����װһ������IJⶨ����������Ʒ���ȵ�ʵ��װ�ã����ѡ������������ (����ĸ)��
A.�٢ڢܢ� B.�٢ܢ� C.�ݢޢ� D.�٢ۢݢ�
(2)ʵ��ѡ���ҩƷ�� ����ѡ��װ�õ�����˳��Ӧ��(����ӿڵ���ĸ�����ӽ���ʡ��) (����ĸ)
(3)д��ʵ����Na2O2��Na2O�ֱ�����Ӧ�����ӷ���ʽ:
��
(4)������ʵ���������Һ���Ƴ�Ũ��Ϊ1.0mol/L����Һ��
����400ml����Һͨ��0.3molCO2����������Һ��C(HCO3-):C(CO32-)ԼΪ �� ��
����A.1:3������������ B.1:2��������������C.2:1��������������D.3:1
����ͬѧ����ѡ������ʵ��װ�û�ʹ�ⶨ���������ƫС����д��һ������Ϊ�Ŀ���
ԭ��
(5)���²�ú�����õ��Ծ���(���ƺ������)��ʢ�ŵ�Ҳ�ǹ������ƣ�д�������Ծ����Ծȹ��ܵĻ�ѧ��Ӧ����ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
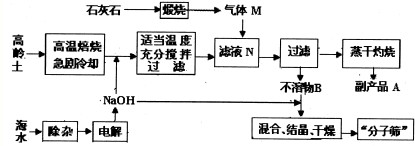
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com