
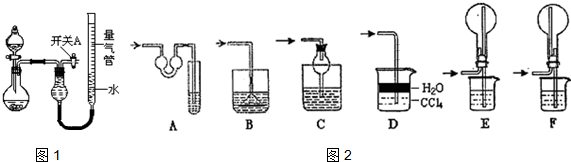
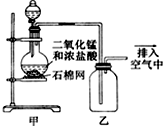
 ŹµŃéŹŅÓƱ½ŗĶÅØĻõĖį”¢ÅØĮņĖį·¢Éś·“Ó¦ÖĘČ”Ļõ»ł±½µÄ×°ÖĆĶ¼ČēĶ¼ĖłŹ¾£®»Ų“šĻĀĮŠĪŹĢā£ŗ
ŹµŃéŹŅÓƱ½ŗĶÅØĻõĖį”¢ÅØĮņĖį·¢Éś·“Ó¦ÖĘČ”Ļõ»ł±½µÄ×°ÖĆĶ¼ČēĶ¼ĖłŹ¾£®»Ų“šĻĀĮŠĪŹĢā£ŗ £®
£®·ÖĪö £Ø1£©Ķ¼ÖŠøų·“Ó¦Īļ¼ÓČȵķ½·ØŹĒĖ®Ō”¼ÓČČ£¬Ė®Ō”¼ÓČȱćÓŚæŲÖĘĪĀ¶Č£¬ŹÜČČ¾łŌČ£»
£Ø2£©ÅäÖĘ»ģŗĻĖįŹ±Ó¦½«ÅØĮņĖį¼ÓČėÅØĻõĖįÖŠ£¬²¢²»¶ĻÕńµ“£¬ŅŌ·ĄÖ¹½¦³öÉĖČĖ£»
£Ø3£©±½ÓėÅØĻõĖįŌŚÅØĮņĖį”¢¼ÓČėĢõ¼žĻĀ·¢ÉśČ”“ś·“Ӧɜ³ÉĻõ»ł±½ÓėĖ®£»
£Ø4£©ÓÉÓŚ±½ŗĶĻõĖį¶¼ŹĒŅ×»Ó·¢”¢ÓŠ¶¾µÄĪļÖŹ£¬Éč¼ĘŹµŃ鏱Ӧæ¼ĀĒĖüĆĒæÉÄܲśÉśµÄĪŪČ¾ŗĶÓɻӷ¢µ¼ÖĀµÄĄūÓĆĀŹ½µµĶ£»
£Ø5£©øĆ·“Ó¦ÖŠÅØĮņĖįµÄ×÷ÓĆ“ß»Æ¼ĮŗĶĪüŹÕ¼Į£®
½ā“š ½ā£ŗ£Ø1£©Ķ¼ÖŠøų·“Ó¦Īļ¼ÓČȵķ½·ØŹĒĖ®ČܼÓČČ£¬Ė®Óņ¼ÓČȱćÓŚæŲÖĘĪĀ¶Č£¬ŹÜČČ¾łŌČ£¬
¹Ź“š°øĪŖ£ŗ±ćÓŚæŲÖĘĪĀ¶Č£»ŹÜČČ¾łŌČ£»
£Ø2£©ÅäÖĘ»ģŗĻĖįŹ±Ó¦½«ĆܶȓóµÄÅØĮņĖį¼ÓČėµ½ĆܶȊ”µÄÅØĻõĖįÖŠČ„£¬ÅäÖĘ»ģŗĻĖįŹ±Ó¦½«ÅØĮņĖį¼ÓČėÅØĻõĖįÖŠ£¬²¢²»¶ĻÕńµ“£¬ŅŌ·ĄÖ¹½¦³öÉĖČĖ£¬
¹Ź“š°øĪŖ£ŗÅØĮņĖį£»ÅØĻõĖį£»
£Ø3£©±½ÓėÅØĻõĖįŌŚÅØĮņĖį”¢¼ÓČėĢõ¼žĻĀ·¢ÉśČ”“ś·“Ӧɜ³ÉĻõ»ł±½ÓėĖ®£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ £¬
£¬
¹Ź“š°øĪŖ£ŗ £®
£®
£Ø4£©ÓÉÓŚ±½ŗĶĻõĖį¶¼ŹĒŅ×»Ó·¢”¢ÓŠ¶¾µÄĪļÖŹ£¬Ó¦æ¼ĀĒĖüĆĒæÉÄܲśÉśµÄĪŪČ¾ŗĶÓɻӷ¢µ¼ÖĀµÄĄūÓĆĀŹ½µµĶ£¬×°ÖĆȱɣĄäÄż»ŲĮ÷×°ÖĆ£¬
¹Ź“š°øĪŖ£ŗ±½”¢ÅØĻõĖįµČ»Ó·¢µ½æÕĘųÖŠ£¬Ōģ³ÉĪŪČ¾£»
£Ø5£©øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬ÅØĮņĖįµÄ×÷ÓĆ“ß»Æ¼ĮŗĶĪüŹÕ¼Į£¬
£¬ÅØĮņĖįµÄ×÷ÓĆ“ß»Æ¼ĮŗĶĪüŹÕ¼Į£¬
¹Ź“š°øĪŖ£ŗ“߻ƼĮ”¢ĪüŹÕ¼Į£®
µćĘĄ ±¾Ģāæ¼²éĻõ»ł±½ÖʱøŹµŃéµÄÓŠ¹ŲÅŠ¶Ļ£¬øĆĢāŹĒ»ł“”ŠŌŹŌĢāµÄ漲飬ŹŌĢā×¢ÖŲ»ł“”£¬Ö»ŅŖŹĒæ¼²éѧɜ¶ŌĻõ»ł±½ÖʱøŹµŃéµÄĮĖ½āÕĘĪÕ³Ģ¶Č£¬ŅŌ¼°Įé»īŌĖÓĆ»ł“”ÖŖŹ¶½ā¾öŹµ¼ŹĪŹĢāµÄÄÜĮ¦£¬ÓŠĄūÓŚÅąŃųѧɜµÄŹµŃéÄÜĮ¦£¬ŗĶŃĻ½÷µÄĀß¼Ė¼Ī¬ÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
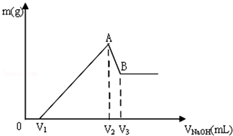 ½«Mg”¢Al×é³ÉµÄ»ģŗĻĪļ¹²0.1molČÜÓŚ100mL 3mol/LHClČÜŅŗÖŠ£¬ŌŁµĪ¼Ó1mol/LNaOH ČÜŅŗ£¬ŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķµÄÖŹĮæmĖęNaOHČÜŅŗĢå»żVµÄ±ä»ÆČēĶ¼ĖłŹ¾£ŗ
½«Mg”¢Al×é³ÉµÄ»ģŗĻĪļ¹²0.1molČÜÓŚ100mL 3mol/LHClČÜŅŗÖŠ£¬ŌŁµĪ¼Ó1mol/LNaOH ČÜŅŗ£¬ŌŚµĪ¼ÓNaOHČÜŅŗµÄ¹ż³ĢÖŠ£¬³ĮµķµÄÖŹĮæmĖęNaOHČÜŅŗĢå»żVµÄ±ä»ÆČēĶ¼ĖłŹ¾£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé | Ņ©Ę· | ÖĘČ”ĘųĢå | ĮæĘų¹ÜÖŠµÄŅŗĢå |
| ¢ń | Cu”¢Ļ”HNO3 | H2O | |
| ¢ņ | NaOH¹ĢĢ唢ÅØ°±Ė® | NH3 | |
| ¢ó | Na2SO3¹ĢĢ唢ÅØH2SO4 | SO2 | |
| ¢ō | Ć¾ĀĮŗĻ½š”¢NaOHČÜŅŗ£Ø×ćĮ棩 | H2 | H2O |
| ±ąŗÅ | Ć¾ĀĮŗĻ½š¶ČĮæ | ĮæĘų¹ÜµŚŅ»“Ī¶ĮŹż | ĮæĘų¹ÜµŚ¶ž“Ī¶ĮŹż |
| ¢Ł | 1.0g | 10.0mL | 346.3mL |
| ¢Ś | 1.0g | 10.0mL | 335.0mL |
| ¢Ū | 1.0g | 10.0mL | 345.7mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
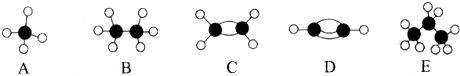
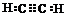 £¬AµÄ¶žĀČČ”“śĪļÓŠ1ÖÖ£®
£¬AµÄ¶žĀČČ”“śĪļÓŠ1ÖÖ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĆ×°ÖĆĶ¼ÖŠÖĮÉŁ“ęŌŚĮ½“¦Ć÷ĻŌ“ķĪó | |
| B£® | ÉÕĘæÖŠµÄMnO2æÉ»»³ÉKClO3»ņKMnO4 | |
| C£® | ŌŚ¼ÆĘųĘæµÄµ¼¹ÜæŚ“¦·ÅŅ»Ę¬ŹŖČóµÄµķ·Ūµā»Æ¼ŲŹŌÖ½æÉŅŌÖ¤Ć÷ŹĒ·ńÓŠĀČĘųŅŻ³ö | |
| D£® | ŌŚŅŅŗóĮ¬Ņ»Ź¢ÓŠ±„ŗĶŹ³ŃĪĖ®µÄÉÕ±æɽųŠŠĪ²Ęų“¦Ąķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ČÜÖŹ | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pHÖµ | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
| A£® | CO2+H2O+NaClOØTNaHCO3+HClO | B£® | CO2+H2O+2NaClOØTNa2CO3+2HClO | ||
| C£® | CH3COOH+NaCNØTCH3COONa+HCN | D£® | CH3COOH+NaClOØTCH3COONa+HClO |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | SO2”¢SiO2”¢CO¾łĪŖĖįŠŌŃõ»ÆĪļ | B£® | Ļ”¶¹½¬”¢¹čĖį”¢ĀČ»ÆĢśČÜŅŗ¾łĪŖ½ŗĢå | ||
| C£® | ÉÕ¼ī”¢“æ¼ī”¢ŹÆÓ¢¾łĪŖµē½āÖŹ | D£® | ĀČĖ®”¢Ė®²£Į§”¢°±Ė®¾łĪŖ»ģŗĻĪļ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com