
| ʵ����� | ��Ʒ����/g | ��������/g |
| 1 | 1.716 | 2.758 |
| 2 | 2.574 | ______ |
| 3 | 3.432 | 5.516 |
| 4 | 4.290 | 5.516 |
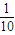 ����ˮ���500mL��Һ����ϡ���Ժ���Һ��pH����Ҫ����д������̣�
����ˮ���500mL��Һ����ϡ���Ժ���Һ��pH����Ҫ����д������̣�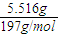 =0.028mol�����ݱ������غ��֪�������������ʵ���Ϊ0.028mol���ݴ˼��㣮
=0.028mol�����ݱ������غ��֪�������������ʵ���Ϊ0.028mol���ݴ˼��㣮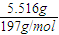 =0.028mol�����ݱ������غ��֪�������������ʵ���Ϊ0.028mol������Ba��OH��2��Һ�����ʵ����ʵ���Ũ����
=0.028mol�����ݱ������غ��֪�������������ʵ���Ϊ0.028mol������Ba��OH��2��Һ�����ʵ����ʵ���Ũ����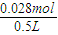 =0.056mol/L��
=0.056mol/L��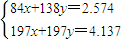 �����x=0.006��y=0.015��
�����x=0.006��y=0.015��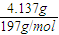 =0.021mol���ɱ������غ��֪����Һ��n[Ba��OH��2]=0.056L/mol×0.5L-0.021mol=0.007mol�����������غ��֪����Һ��n��NaOH��=n��NaHCO3��=0.006mol���ɼ������غ��֪����Һ��n��KOH��=2n��K2CO3��=2×0.015mol=0.03mol��������Һ��n��OH-��=2n[Ba��OH��2]+n��NaOH��+n��KOH��=0.007mol×2+0.006mol+0.03mol=0.05mol��ȡ������Һ�����
=0.021mol���ɱ������غ��֪����Һ��n[Ba��OH��2]=0.056L/mol×0.5L-0.021mol=0.007mol�����������غ��֪����Һ��n��NaOH��=n��NaHCO3��=0.006mol���ɼ������غ��֪����Һ��n��KOH��=2n��K2CO3��=2×0.015mol=0.03mol��������Һ��n��OH-��=2n[Ba��OH��2]+n��NaOH��+n��KOH��=0.007mol×2+0.006mol+0.03mol=0.05mol��ȡ������Һ�����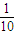 �����е������������ʵ���Ϊ0.005mol����ˮ���500mL��Һ����������Ũ��Ϊ
�����е������������ʵ���Ϊ0.005mol����ˮ���500mL��Һ����������Ũ��Ϊ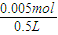 =0.01mol/L������c��H+��=10-12mol/L��������Һ��pH=-log10-12=12��
=0.01mol/L������c��H+��=10-12mol/L��������Һ��pH=-log10-12=12��

 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ��ȡ��Ʒ����/g | ����Ba��OH��2��Һ�����/L | ������ɳ���������/g |
| 1 | 0.858 | 0.5 | 1.379 |
| 2 | 1.716 | 0.5 | �� |
| 3 | 2.574 | 0.5 | 4.137 |
| 4 | 3.432 | 0.5 | 5.516 |
| 5 | 4.290 | 0.5 | 5.516 |
| 6 | 5.148 | 0.5 | 5.516 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ��ȡ��Ʒ������/g | ����Ba��OH��2��Һ�����/L | ������ɳ�������/g |
| 1 | 0.858 | 0.5 | 1.379 |
| 2 | 1.716 | 0.5 | 2.758 2.758 |
| 3 | 2.574 | 0.5 | 4.137 |
| 4 | 3.432 | 0.5 | 5.516 |
| 5 | 4.290 | 0.5 | 5.516 |
| 6 | 5.148 | 0.5 | 5.516 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ��Ʒ����/g | ��������/g |
| 1 | 1.716 | 2.758 |
| 2 | 2.574 | 4.137 4.137 |
| 3 | 3.432 | 5.516 |
| 4 | 4.290 | 5.516 |
| 1 |
| 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ��ȡ��Ʒ������/g | ����Ba��OH��2��Һ�����/L | ������ɳ���������/g |
| 1 | 0.858 | 0.5 | 1.379 |
| 2 | 1.716 | 0.5 | 2.758 2.758 |
| 3 | 2.574 | 0.5 | 4.137 |
| 4 | 3.432 | 0.5 | 5.516 |
| 5 | 4.290 | 0.5 | 5.516 |
| 6 | 5.148 | 0.5 | 5.516 |
| 1 |
| 100 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����� | ��Ʒ����/g | ��������/g |
1 | 1.716 | 2.758 |
2 | 2.574 |
|
3 | 3.432 | 5.516 |
4 | 4.290 | 5.516 |
����������⣺
��1����2��ʵ���в��������������Ƕ��٣�������������հ״���
��2��Ba(OH)2��Һ�����ʵ����ʵ���Ũ����___________��
��3����2��ʵ����Ʒ��NaHCO3�����ʵ�����_________��
��4��������ȡ��2��ʵ��������Һ�����1/10����ˮ���500 mL��Һ����ϡ���Ժ���Һ��pH��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com