
 =300mL��
=300mL��
 ��
��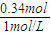 =340mL��
=340mL��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�������ʡ��һ�и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
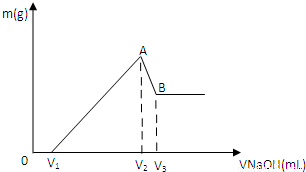
��0.1molMg��Al���������100mL4mol/L�������У�Ȼ���ٵμ�1mol/L��NaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У���������m��NaOH��Һ�����V�ı仯��ͼ��ʾ��
��1��д��BC���̵����ӷ�Ӧ����ʽ
��2����V1=140mLʱ���������n(Mg)=_____mol��V2=_____mL
��3�������NaOH��Һ_____mLʱ,��Һ�е�Mg2+��Al3+�պó�����ȫ��
(4)���������Mg�����ʵ�������Ϊa���������NaOH��ҺΪ450mLʱ�����ó�������Al(OH)3����a��ȡֵ��Χ��____________________
(5)������V2 mlNaOH��Һ����ˣ�����Һ��ͨ�������CO2���壬�ܹ۲쵽������Ϊ ��д���ù��̷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ˮ��һ�и�����һ��ѧ�ο��Ի�ѧ�Ծ����������� ���ͣ�������
(8��) ��0.1molMg��Al���������100mL4mol/L�������У�Ȼ���ٵμ�1mol/L��NaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У���������m��NaOH��Һ�����V�ı仯��ͼ��ʾ��
(1)��V1=140mLʱ���������n(Mg)=_____mol��V2=_____mL
(2)�����NaOH��Һ_____mL ʱ,��Һ�е�Mg2+��Al3+�պó�����ȫ��
(3)���������Mg�����ʵ�������Ϊa���������NaOH��ҺΪ450mLʱ�����ó�������Al(OH)3����a��ȡֵ��Χ��____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��0.1molMg��Al���������100mL4mol/L�������У�Ȼ���ٵμ�1mol/L��NaOH��Һ���ڵμ�NaOH��Һ�Ĺ����У���������m��NaOH��Һ�����V�ı仯��ͼ��ʾ��
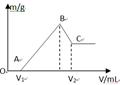
��1��д��BC���̵����ӷ�Ӧ����ʽ
��2����V1=140mLʱ���������n(Mg)=_____mol��V2=_____mL
��3�������NaOH��Һ_____mLʱ,��Һ�е�Mg2+��Al3+�պó�����ȫ��
(4)���������Mg�����ʵ�������Ϊa���������NaOH��ҺΪ450mLʱ�����ó�������Al(OH)3����a��ȡֵ��Χ��____________________
(5)������V2 mlNaOH��Һ����ˣ�����Һ��ͨ�������CO2���壬�ܹ۲쵽������Ϊ ��д���ù��̷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com