
��100 g̼����ȫȼ�����������У�CO��CO2�������Ϊ1��2����֪��
C(s)��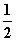 O2(g)===CO(g)����H1����110.35 kJ·mol��1��
O2(g)===CO(g)����H1����110.35 kJ·mol��1��
CO(g)��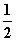 O2(g)===CO2(g)����H2����282.57 kJ·mol��1��
O2(g)===CO2(g)����H2����282.57 kJ·mol��1��
����100 g̼��ȫȼ����ȣ���ʧ��������(����)
A��392.92 kJ B��2 489.42 kJ C��784.92 kJ D��3 274.3 kJ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����β������ɴ�����Ⱦ����Ҫԭ��֮һ,�������������ϰ�װ����ת������ʹ������β��ת������������Ŀǰ����Ч���ֶ�֮һ������� ��ʾ̼ԭ��,��
��ʾ̼ԭ��,�� ��ʾ��ԭ��,��
��ʾ��ԭ��,�� ��ʾ��ԭ��,��ͼΪ����ת�����۹��̡��������ͼʾ�ش���������:
��ʾ��ԭ��,��ͼΪ����ת�����۹��̡��������ͼʾ�ش���������:
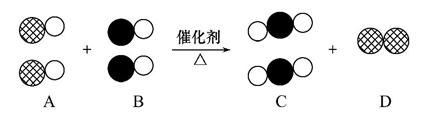
(1)A��B��C�������ʿ��Թ�Ϊһ��������� ����
(2)��C��Ϊ������,��D��Ϊ���ʵ������� ����
(3)�û�ѧ��Ӧ����ʽ��ʾΪ�� ��
��ѧ�仯���������ĵ�A���ʺ����ɵ�C���ʵ�������Ϊ����������
(4)���۵ĽǶ�ȥ�������õĹ��ڻ�ѧ�仯���й���Ϣ(���һ������)
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����о���ѧ�Ļ������������и�װ��ͼ�������У���ȷ����

A.װ�âٳ�����ʵ������ȡ��
B.װ�â���X��Ϊ�������������հ���������ֹ����
C.װ�âۿ������Ʊ��������������۲�����ɫ
D.װ�âܿ���֤HCl������ˮ�е��ܽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ�������Ʊ��������֤�������ʵ�װ��ͼ
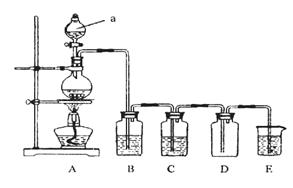
(1)д����A��ȡ�����Ļ�ѧ����ʽ_________________________________________��
(2)������ͼװ���Ʊ���������������֤�������ԣ�Cl2>Fe3+
��װ��B�е���Һ��������___________________________________��
װ��D�мӵ�����Լ���(�����)___________��
��ѡ�Լ���a��ŨH2SO4 b��FeCl2��Һ c��KSCN��FeCl2�Ļ����Һ d����ˮ�Ȼ���
(3)����ͼ��ʾԲ����ƿ�ڼ���̼��a�м���Ũ���ᣬ��ʼʵ�飬���Ȳ��������建��ͨ������װ��ͬʱ�������ʵ�飺
ʵ��1��֤��SO2���������Ժ�Ư����
ʵ��2��֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ
֤��SO2�������Ժ�Ư���ԣ�B��Ϊ����Na2S��Һ��C�м�Ʒ����Һ��D��Ӧ����������__________(����Һ����)��E�м���___________��Һ(�ѧʽ)��
(4)֤��̼Ԫ�صķǽ����Աȹ�Ԫ�ص�ǿ������Ϊ______________________��
ʵ��2���Ͻ�֮��Ӧ��θĽ�________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����ǵ�ȼ�����Ǧ�H����2 840 kJ·mol��1��������������1 gҺ̬ˮʱ�ų�����
����(����)
A��26.3 kJ���� B��51.9 kJ C��155.8 kJ���� D��467.3 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)��H2O(g)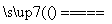 CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g)��H2(g)��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽΪ��
C(s)��O2(g)===CO2(g) ��H����393.5 kJ·mol��1
H2(g)��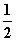 O2(g)===H2O(g) ��H����242.0 kJ·mol��1
O2(g)===H2O(g) ��H����242.0 kJ·mol��1
CO(g)��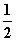 O2(g)===CO2(g) ��H����283.0 kJ·mol��1
O2(g)===CO2(g) ��H����283.0 kJ·mol��1
��ش�
(1)�����������ݣ�д��C(s)��ˮ������Ӧ���Ȼ�ѧ��Ӧ����ʽ��________________________________________________________________________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��������ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹������������ѭ��ͼ��
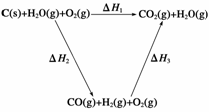
���ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
��������ס�����ͬѧ�۵���ȷ����__________(��ס����ҡ�)���жϵ�������________________________________________________________________________
________________________________________________________________________��
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ㣺________________________________________________________________________
________________________________________________________________________��
(4)ˮú������������������ȼ�ϣ�Ҳ����Ҫ���л�����ԭ�ϡ�CO��H2��һ�������¿��Ժϳɣ��ټ״����ڼ�ȩ���ۼ��ᡡ�����ᡣ�Է�����CO��H2��1��1������Ȼ�Ϸ�Ӧ���ϳ�����________(�����)����ʱ���������㡰��ɫ��ѧ����Ҫ����ȫ����ԭ���е�ԭ�ӣ�ʵ�����ŷš�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��25��C��100 kPa�£�1 g�״�ȼ������CO2��Һ̬ˮʱ����22.68 kJ�������Ȼ�ѧ����ʽ��ȷ����(����)
A��CH3OH(l)��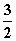 O2(g)===CO2(g)��2H2O(l) ��H��725.8 kJ·mol��1
O2(g)===CO2(g)��2H2O(l) ��H��725.8 kJ·mol��1
B��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l) ��H����1 452 kJ·mol��1
C��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l) ��H����725.8 kJ·mol��1
D��2CH3OH(l)��3O2(g)===2CO2(g)��4H2O(l) ��H��1 452 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��пƬ��ͭƬΪ��������ϡ����Ϊ�������Һ���ԭ��أ���������ͨ��2 mol����ʱ������˵����ȷ����(����)
A��пƬ�ܽ���1 mol��ͭƬ������1 mol H2
B���������ܽ�������������������
C��пƬ�ܽ�31 g��ͭƬ������1 g H2
D��пƬ�ܽ���1 mol����������0.5 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڹ̶��ܱ������У����淴Ӧ2X(g)+2Y(s) 3Z(g)���ﵽƽ�������������X��Y��Z�����ʵ���������һ�룬�Ը÷�Ӧ������Ӱ����( )
3Z(g)���ﵽƽ�������������X��Y��Z�����ʵ���������һ�룬�Ը÷�Ӧ������Ӱ����( )
A�������淴Ӧ���ʶ���С��ƽ�ⲻ�ƶ�
B�������淴Ӧ���ʶ���С��ƽ��������Ӧ�����ƶ�
C������Ӧ���������淴Ӧ���ʼ�С��ƽ��������Ӧ�����ƶ�
D������Ӧ���ʼ�С���淴Ӧ��������ƽ�����淴Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com