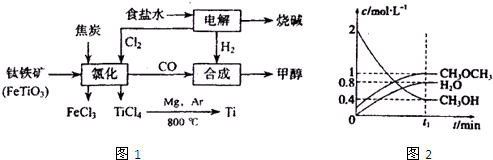
�ѣ�Ti������Ϊ��������֮��ĵ���������Ҳ����˵21�������ѵ����ͣ����ڵؿ��еĺ��������٣����ѵ�ұ��������δ���ͻ�ƣ�Ŀǰ��ֻ���ڼ��������ͼ1��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ���ɴ�������Դ�����ʣ����ٻ�����Ⱦ��
����д���пհף�
��l���ö��Ե缫���2Lʳ��ˮʱ���ܷ�Ӧ�����ӷ���ʽ
���������ϲ���224mL���壨��״����ʱ��������Һ��pH=
��������ǰ����Һ������䣬ʳ��ˮ��������
��2��д���������������Ȼ��õ����Ȼ��ѵĻ�ѧ����ʽ
������ʾ��FeTiO
3��TiΪ+4�ۣ�
��3����Ӧ2Mg+TiCl
42MgCl
2+Ti��Ar������������
��
��4����������һ����Ҫ�����ȼ�ϣ�����ͨ���״����Ӽ���ˮ�Ƶã�2CH
3OH��g��?CH
3OCH
3��g��+H
2O��g����H=-23.5kJ/mol
T
1��ʱ���ں����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ2��ʾ��
��T
1��ʱ���÷�Ӧ��ƽ�ⳣ��Ϊ
��
����ͬ�����£����ı���ʼŨ�ȣ�ijʱ�̸����Ũ������Ϊ��c��CH
3OH��=0.4mol/L��c��H
2O��=0.6mol/L��c��CH
3OCH
3��=1.2mol/L����ʱ�����淴�����ʵĴ�С��v��
v�棨���������������=������
��5����������ҵ���У��ϳ�192�ּ״�����������ⲹ��H
2
�֣��������������������ʵ��κ���ʧ����





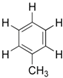 �����ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ������ȡ�������ܵõ���һԪȡ�����У�������
�����ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ������ȡ�������ܵõ���һԪȡ�����У�������

 ʵ��������ͼװ����ȡ����������
ʵ��������ͼװ����ȡ����������