
| ���� | �е� | �۵� | �ܽ��� |
| ����ȩ | 179�� | -26�� | ����ˮ���������ѻ��ܣ� |
| ���״� | 205.3�� | -15.3�� | ������ˮ���������ѻ��� |
| ������ | 249�� | 122�� | ����ˮ�����������ѣ� |
| ���� | 34.8�� | ������ˮ |

| ��� | ʵ�鷽�� | ʵ������ | ���� |
| ����� | ȡ������Ʒ���ڽྻ���Թ��У��ټ���2mLAg��NH3��OH ��Һ��ˮԡ���ȣ� | �Թ��ڱڲ����������� | ��Ʒ���к��б���ȩ |
| ����� | ��������Ʒ�����ڷ�Һ©���У�Ȼ����©���м��뱥��NaHSO3��Һ����Һ����ַ�Ӧ���Һ�� | ||
| ����� | ������ڵ��л���ϴ�ӡ�����ⶨ��Ʒ�� �е� | �е�Ϊ205.3�� | �����л����Ǵ����ı��״� |
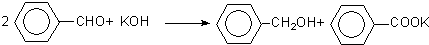
���� �����̿�֪������ȩ��KOH��Ӧ���ɱ��״���������أ�Ȼ���ˮ��������ȡ���״���������ֲ�������ˮ��Һ����Ϊ��ȡ��Һ����������Һ�к����״�������IIΪ���õ���Ʒ��Ϊ���״���ˮ��Һ�к�������أ������ᷢ��ǿ����ȡ����ķ�Ӧ�����ɱ����ᣬ��������ܽ��С���������Ϊ���ˣ����Ʒ��Ϊ�����ᣬ������ᴿ���״��뱽��ȩ�Ļ���������������Ӧ���鱽��ȩ���÷�Һ�ķ��������ᴿ�ⶨ���״��ķе���鱽�״��Ĵ��ڣ����ݱ��״�����������=$\frac{��Ʒ������-����ȩ������}{��Ʒ������}$��100%���㣬�Դ������
��� �⣺�����̿�֪������ȩ��KOH��Ӧ���ɱ��״���������أ�Ȼ���ˮ��������ȡ���״���������ֲ�������ˮ��Һ����Ϊ��ȡ��Һ����������Һ�к����״�������IIΪ���õ���Ʒ��Ϊ���״���ˮ��Һ�к�������أ������ᷢ��ǿ����ȡ����ķ�Ӧ�����ɱ����ᣬ��������ܽ��С���������Ϊ���ˣ����Ʒ��Ϊ�����ᣬ
��1����������ķ�����֪������I�������Ƿ�Һ������II������������
�ʴ�Ϊ����Һ������
��2����������ķ�����֪������III�������ǹ��ˣ���Ʒ���DZ����ᣬ
�ʴ�Ϊ�����ˣ������
��3��������ᴿ���״��뱽��ȩ�Ļ���������������Ӧ���鱽��ȩ���÷�Һ�ķ��������ᴿ�ⶨ���״��ķе���鱽�״��Ĵ��ڣ����Բ����ȡ������Ʒ���ڽྻ���Թ��У��ټ���2mLAg��NH3��OH��Һ��ˮԡ���ȣ��Թ��ڱڲ����������������Ʒ���к��б���ȩ������ڽ�������Ʒ�����ڷ�Һ©���У�Ȼ����©���м��뱥��NaHSO3��Һ����ַ�Ӧ���Һ������۽�����ڵ��л���ϴ�ӡ������ⶨ��Ʒ�ķе㣬�е�Ϊ205.3�棬����˵�������л����Ǵ����ı��״���
�ʴ�Ϊ��2mLAg��NH3��OH��Һ���Թ��ڱڲ�����������������NaHSO3��Һ����Һ���ⶨ��Ʒ�ķе㣻�е�Ϊ205.3�棻
��4����ȡ7.00g��Ʒ�ף���������������Һ��ַ�Ӧ�����ɵ���Ag 2.16g�����ݹ�ϵʽ����ȩ��2Ag����֪����ȩ������Ϊ$\frac{2.16}{108}��\frac{1}{2}��106$g=1.06g�����Ա��״�����������=$\frac{��Ʒ������-����ȩ������}{��Ʒ������}$��100%=$\frac{7.00g-\frac{2.16g}{108g/mol}��\frac{1}{2}��106g/mol}{7.00g}$100%=$\frac{7-1.06}{7}$��100%=84.9%��
�ʴ�Ϊ��$\frac{7.00g-\frac{2.16g}{108g/mol}��\frac{1}{2}��106g/mol}{7.00g}$100%��84.9%��
���� ���⿼���л���ķ����ᴿ����ؼ��㣬Ϊ��Ƶ���㣬�����л�������ʼ����������еķ�Ӧ���������뷽��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 +2nH2O��
+2nH2O�� +2NaOH$��_{��}^{H_{2}O}$CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��
+2NaOH$��_{��}^{H_{2}O}$CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 50% | B�� | 36% | C�� | 64% | D�� | 45% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڶ���ƽ��ʱBΪ��̬ | |
| B�� | a��3 | |
| C�� | ��һ��ƽ�������ѹǿƽ�������ƶ� | |
| D�� | �����δﵽƽ��ʱB����Ϊ����̬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ϩ���ϵ��ϻ�����Ϊ�����˼ӳɷ�Ӧ | |
| B�� | ú����������Һ���������仯��ת��Ϊ���ȼ�� | |
| C�� | �����ǡ���֬�������ʶ���ˮ�⣬��ˮ����ﲻͬ | |
| D�� | ������ʳ��ƾ����˵��ۡ������ǡ��Ҵ��Ļ�ѧ�仯���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2 | B�� | SO3 | C�� | HCl | D�� | FeSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Ũ������ | B�� | ���뼸��CuSO4��Һ | ||
| C�� | ����CH3COONa���� | D�� | ������Ƭ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ�����������ԣ�ϡ������������ | |
| B�� | ������������������ʢ��Ũ���� | |
| C�� | Ũ�����ڳ����¿�Ѹ����ͭƬ��Ӧ�ų�һ���������� | |
| D�� | Ũ�����Ҫ����ɫ�Լ�ƿ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com