
��1��ʵ�����ɴ�����ͭм�Ƶ�����CuSO4��5H2O����ʵ���������£�
�ܽ�ͭмһ�ַ����ǣ���ͭм���뵽ϡ������˫��ˮ�Ļ��Һ�в���30��40��ˮԡ���ȣ�һ��ʱ���ͭ��ȫ�ܽ⣬�õ�����ͭ��Һ��
�ٸ÷�Ӧ�Ļ�ѧ����ʽΪ ��
�ڷ�Ӧ�¶Ȳ��ܳ���40���ԭ���� ��
��������ͭ��Һ��õ����IJ�������Ϊ ����ȴ�ᾧ�� ��ϴ�ӡ����
��2��Ŀǰ�ҹ��Ļ����������������Ϊȼú���飬����ȼúΪ���ĵ�����������ɵĻ�����Ⱦ����Լ������ҵ��չ��һ����Ҫ���أ����е������NOx���Ǽ̷۳��Ͷ�������֮��ȼú��վ�����������ص㡣
��ȼú��������ķ����ܶ࣬��ʯ��ʯ��ʯ�෨����ˮ���ȡ�����ʯ��ʯ-ʯ�෨�����ԭ����һ����SO2+Ca(OH)2=CaSO3+H2O��Ȼ���ٽ����������Ƴ�ʯ��,д���÷�Ӧ�Ļ�ѧ����ʽ ��
��ȼú���������ɲ��ð���NH3����Ϊ��ԭ���ʣ��ڴ������ڵ������£���������(NOx)�뻹ԭ��������Ӧ���������ĵ�����ˮ��д�����������백��Ӧ�Ļ�ѧ����ʽ ��
��1����H2SO4+H2O2+Cu=CuSO4+2H2O;
�ڷ�ֹ��������ķֽ⣻
������Ũ��������
��2����2CaSO3+O2=2CaSO4������CaSO4��H2O��CaSO4��2H2O��ƽҲ���֣�
��6NO2+8NH3=7N2+12H2O
���������������1����ͭ��ϡ���ᡢ˫��ˮ����������ԭ��Ӧ����������ͭ��Һ����ѧ����ʽΪH2SO4+H2O2+Cu=CuSO4+2H2O;
�ڹ������ⲻ�ȶ��������ֽ⣬���Է�Ӧ�¶Ȳ��ܳ���40��
������Һ�õ�����IJ���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����
��2����ʯ��Ϊ����ƵĽᾧˮ������������������Ӧ�ɵ�ʯ�࣬��ѧ����ʽΪ2CaSO3+O2=2CaSO4
�ڶ��������백��Ӧ�������ĵ�����ˮ,��ѧ����ʽΪ6NO2+8NH3=7N2+12H2O
���㣺����Ի�ѧʵ��ķ������������жϣ���ѧ����ʽ����д



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���Խ�������ɫҺ�壺C2H5OH��AgNO3��Һ��C2H5Br��KI��Һ��C6H5OH��Һ��C6H6���ֿ����Լ���
| A��FeCl3��Һ | B��ϡ���ᡡ |
| C�����Ը��������Һ | D��NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
V2O5�ǽӴ������������Ҫ��������ҵ������V2O5�Ĺ����������£���ش��������⣺
��1����������÷����ijɷ��� (д��ѧʽ)������NaOH��Һ��Ӧ�����ӷ�Ӧ����ʽΪ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ(��ʽR��ʾVO2+��HA��ʾ�л���ȡ��)��
R2(SO4)n(ˮ��)+2nHA(�л���) 2RAn(�л���)+nH2SO4(ˮ��)��
2RAn(�л���)+nH2SO4(ˮ��)��
��ʵ�����в���ڡ���ʹ�õ���Ҫ������ ��
������ȡʱ��������������ԭ���� ��
��3���������X�Լ�Ϊ ������ܵ�Ŀ���� ������ݵ����ӷ���ʽΪ ��
��4���ù��������У�����ѭ�����õ������� �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϳ��ø�������Ч�ɷ�ΪFeO��Cr2O3����Ҫ����ΪSiO2��Al2O3��Ϊԭ�������ظ���أ�K2Cr2O7����ʵ����ģ�ҵ���ø��������ظ���ص���Ҫ������������ͼ���漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3��24NaOH��7KClO3=12Na2CrO4��3Fe2O3��7KCl��12H2O���Իش��������⣺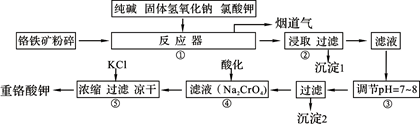
��1��������Һ���������ӵļ��鷽���� ��
��2������۱�����������Ϊ�������ӷ��ţ� ��
��3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4���̵����е�CO2����H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H=��725.5 kJ/mol����H=��285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��5��2011�����������ĸ���Ⱦ�¼���˵��������������ˮ���������ŷŶ��������滷���м����Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ�������������������ʯī��������⺬Cr2O72-�����Է�ˮ��һ��ʱ������Fe(OH)3��Cr(OH)3������
��д����ⷨ������ˮ���ܷ�Ӧ�����ӷ���ʽ ��
����֪Cr(OH)3��Ksp=6.3��10�C31�����ر�ˮ�����������ֵ��0.1 mg/L��Ҫʹ��Һ��c(Cr3+)�������ϵر�ˮ��ֵ���������Һ��c(OH-)�� mol/L��ֻд�������ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ǧ�㷺Ӧ�����������ء�����пұ�������е�Ǧ������������Ǧ���������£�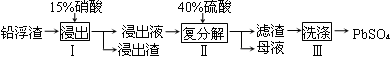
��֪Ǧ��������Ҫ�ɷ���PbO��Pb������������Ag��Zn��CaO��������������������ʡ�25��ʱ��Ksp(CaSO4)��4.9��10��5��Ksp(PbSO4)��1.6��10��8��
��1����֪�������NO����������Һ�к���������������Pb2+���ֱ�д��PbO��Pb�μӷ�Ӧ�����ӷ���ʽ �� ��
��2�����������������������ʹPb����ʣ�࣬Ŀ���� ��
��3��ĸҺ��ѭ�������ڲ������������Ҫ�� (��һ�����ʻ�ѧʽ)����ĸҺ�в�����SO42�����࣬ѭ������ʱ���ܳ��ֵ������� ��
��4����ƷPbSO4������Pb(NO3)2��Һ���ϴ�ӣ�Ŀ���dz�ȥ ��
��5��Ǧ���صĵ��Һ�����ᣬ���������缫�ϳ�����PbSO4�ֱ�ת��ΪPbO2��Pb�����ʱ�����ĵ缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�������ӹ����θ�д����������ʽ��KAlSi3O8�� ��
��2����һ����ɫ��ĩ������K2SO4��NaHCO3��BaCl2��FeCl3��KCl���������е�ij������ɣ��ֽ�������ʵ�飺
�ٽ���ɫ��ĩ��ˮ�ܽ⣬����ɫ��Һ��
����������õ���ɫ��Һ�м���NaOH��Һ���۲쵽�а�ɫ����A���ɣ����˺�����Һ�еμ������ữ��AgNO3��Һ���ֵõ���ɫ����B��
�������������жϣ�
��A�Ļ�ѧʽ�� B�Ļ�ѧʽ�� ��
��ԭ��ɫ��ĩ��һ������ �����ܺ��� ��
�Կ��ܺ��е����ʣ���ͨ�� ����ʵ�����ƣ���һ�����顣������������У���պȡ����Һ�������ھƾ��ƻ��������գ�������ɫ�ܲ����۲죻����ϡ����ϴ����˿��������ȷ�IJ���˳��Ϊ ��
A���٢ڢۢ� B���ܢ٢ڢ� C���ܢڢ٢ڢۢ� D���٢ۢڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ��ɫ����Һ����ȷ���Ƿ����������ӣ� Fe2����Mg2����Al3����Ba2����NO3-��SO42-��Cl����I����HCO3-��ȡ����Һ����ʵ�飺
| ʵ�鲽�� | ʵ������ |
| (1)ȡ��������Һ���Ӽ�����ɫʯ����Һ | ��Һ��� |
| (2)ȡ��������Һ���ȣ���CuƬ��ŨH2SO4������ | ����ɫ���������������������ɺ���ɫ |
| (3)ȡ��������Һ����BaCl2��Һ | �а�ɫ���� |
| (4)ȡ(3)���ϲ���Һ����AgNO3��Һ | �а�ɫ�������Ҳ�����ϡHNO3 |
| (5)ȡ��������Һ����NaOH��Һ | �а�ɫ������NaOH����ʱ���������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�ϳ��ú�����Al2O3�ķ�����FeO��V2O5�����۷���ȡV2O5����Ҫ�������£�
��֪���ٱ���ʱAl2O3��V2O5�����봿�Ӧ���������Ӧ�����Σ�ͬʱ�ų�CO2����
�ڳ��������ʵ��ܽ�ȣ�NaVO3��21��2g��l00gˮ��HVO3��0��008g/l00gˮ
��ش𣺣�1����д����������ʱ��V2O5�봿�Ӧ��ѧ����ʽ____ ��
��������B������Ҫ�ɷֿ����Ǣ� ����_ ___ ����_ ___����____ ����д��ѧʽ���ɲ�������
��2�������У���ֱ����H2SO4���ݡ�����A����ȡHVO3��ԭ����____ ��
��3���������١����� ��ϴ�ӡ���������ϴ�ӣ����Ʒ�п��ܺ��еĽ����������� �� ������װ�ã����ּг�����ʡȥ��������ʵ���ҽ��С������ڡ�����____ ��������ţ�
��4��NaVO3����ԭ�͵�����������V2O5������NaOH��Һ����ȡ����Ӧ�����ӷ���ʽΪ____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������,��Ŀǰ����ˮ���塱������Ҫ����֮һ���乤����������:
(1)�������ڱ���λ�����������������������塣
(2)��������������ữ�����Cl2��������,������
(3)�����������SO2�Ļ�ԭ��,��Ӧ�����ӷ���ʽΪ
(4)��������������,�¶�Ӧ������80~90 ��,�¶ȹ�����Ͷ�����������,�����ԭ���� ��
(5)�������������������õ�Һ������ˮ�Ļ����,���������ǵ��ܶ����ܴ���ص���з��롣������������������ ��
(6)����١���֮��δֱ���á���Br2�ĺ�ˮ����������õ�Һ��,���Ǿ�����������������SO2���ա�����������������,������������������ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com