
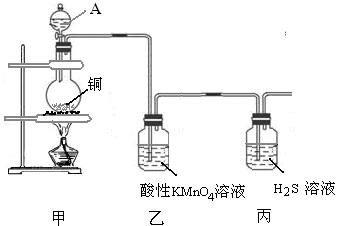
 Cu SO4+SO2��+2H2O��2����Һ��ɫ B
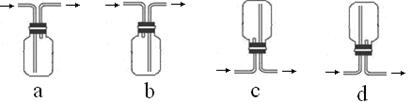
Cu SO4+SO2��+2H2O��2����Һ��ɫ B CuSO4+ SO2��+2H2O��Cu������ƿ�У�Ũ����ͨ����Һ©�����롣������SO2�����л�ԭ�ԡ������ԡ�Ư���Լ����ԡ�������KMnO4��Һ��֤�仹ԭ�ԣ���������ɫ��dz����ɫ����Ӧ�ķ���ʽΪ��2KMnO4+5SO2+H2O=K2SO4+2MnSO4+2H2SO4 .��H2S��ˮ��Һ����֤�������ԣ������Dz�������ɫ��������Ӧ�ķ���ʽΪ2H2S+ SO2=3S��+2H2O . SO2�����ܽ���ˮ�����Բ�������ˮ���ռ���ֻ�����ſ������ռ������������ܶȱȿ���������ֻ���������ſ������ռ�������ѡ��װ��a��c���ռ���SO2�Ǵ�����Ⱦ�ֱ���ŷŻᵼ�´�����Ⱦ�����꣬������ʵ��װ�õ����װһ��β������װ�ã���������NaOH��Һ������β����2������Ʊ����ռ������ʵ���֤��β��������֪ʶ��
CuSO4+ SO2��+2H2O��Cu������ƿ�У�Ũ����ͨ����Һ©�����롣������SO2�����л�ԭ�ԡ������ԡ�Ư���Լ����ԡ�������KMnO4��Һ��֤�仹ԭ�ԣ���������ɫ��dz����ɫ����Ӧ�ķ���ʽΪ��2KMnO4+5SO2+H2O=K2SO4+2MnSO4+2H2SO4 .��H2S��ˮ��Һ����֤�������ԣ������Dz�������ɫ��������Ӧ�ķ���ʽΪ2H2S+ SO2=3S��+2H2O . SO2�����ܽ���ˮ�����Բ�������ˮ���ռ���ֻ�����ſ������ռ������������ܶȱȿ���������ֻ���������ſ������ռ�������ѡ��װ��a��c���ռ���SO2�Ǵ�����Ⱦ�ֱ���ŷŻᵼ�´�����Ⱦ�����꣬������ʵ��װ�õ����װһ��β������װ�ã���������NaOH��Һ������β����2������Ʊ����ռ������ʵ���֤��β��������֪ʶ��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
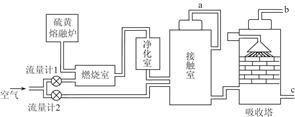
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ɽ�緢�����Ļ�ɽ���в����ܺ���SO3���� |
| B�������ʵ�����SO2��Cl2ͬʱͨ��ˮ�У�������Һ��Ư������ǿ��pH��С |
| C��BaSO3�ܹ�����ϡ���ᣬ���Կ�����Ba(NO3)2��Һ��ϡ�������SO2��SO3 |
| D��һ�������£��������������������Ӧ��ˮ�������Һ��Ӧ�����Եõ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������Ǽ���Ũ������ڣ�˵��Ũ���������ˮ�� |
| B��Ũ�������������������˵��Ũ���������ˮ�� |
| C��ͭ��Ũ���Ṳ���д̼�����ζ����ų���˵��Ũ�������ǿ������ |
| D��������Ũ��������������棬˵���������Ũ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������Ư���ԣ����Կ���ʹ��ˮ��ɫ |
| B����������������ȼ�����ɶ��������ڹ���������ȼ�������������� |
| C�������쵪Ԫ�ؿ��Խ�������ת����N2��NO��NO2��HNO3 |
| D�������Ļ�ѧ���ʷdz��ȶ�����֧���κ����ʵ�ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��Fe��Cl2�е�ȼ�ղ��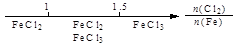 |
B����ˮ��SO2��Ӧ����Һ�е���Σ� |
C��ƽ�ⳣ����ת���ʹ�ϵ�� |
D��Cl2��CH4ȡ����Ӧ��IJ��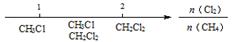 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���ϼ� | -2 | -1 | 0 | +2 | +4 | +6 | +7 |
| ������Ļ�ѧʽ | | FeS2 | S | Na2S2O3 | | SO3��H2SO4��Na2SO4 | Na2S2O8 |
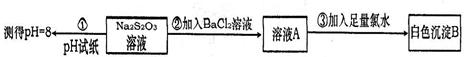
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
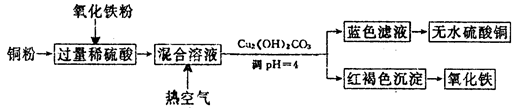
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com