
����Ŀ��ij��ѧʵ������Ҫ0.2mol/L NaOH��Һ500mL��0.5mol/L������Һ450mL��������������Һ����������ش��������⣺
��1����ͼ��ʾ��������������Һ�϶�����Ҫ����������ţ�������������Һ�����õ��IJ��������������������ƣ��� 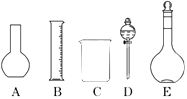
��2������ƿ��������Һ����Ҫ����������ƿ�ϱ������������е�����д��ţ���
���¶� ��Ũ�� ������ ��ѹǿ ����ʽ���ʽ �̶���
��3������ʱ������ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ50mL�����ò���������������ʹ�����ܽ⣬��ȴ������
C��������ƿ�ǽ���ҡ��
D�����ܽ������������Һ�ز�����ע������ƿ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ��ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
��4�����ݼ��㣬����0.2mol/L NaOH��Һ500mL�� NaOH���������Ϊ��g��
��5�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ���������ǣ� ��
��6���������Ƶ�ϡH2SO4���вⶨ������ʵ��Ũ��С��0.5mol/L���������������Щ��������������Ũ��ƫС����д��ĸ�� ��
A.����Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶�
B.����ƿδ���T����������Һ
C.Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж���
D.������ƿת��ʱ��������Һ�彦��
E.������ƿ�ж���ʱ��������ƿ�̶���
F.�ձ�δ����ϴ��
G.���ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�
���𰸡�
��1��AD������������ͷ�ι�
��2���٢ۢ�
��3��B��D��A��F��E��C
��4��4.0
��5����Ũ���������ձ��ڻ���ע��ˮ�У�ͬʱ�����ò���������
��6��BFG
���������⣺��1��������Һ�IJ������裺���ȼ������Ҫ�����ʵ�������Ũ�����������Ȼ����ƽ��������Ͳ��ȡ����������ձ����ܽ⣨ϡ�ͣ���ͬʱ�ò��������裬����Һ��ȴ�����º��ò�����������Һ��500ml����ƿ��Ȼ��ϴ���ձ��Ͳ�����2��3�Σ���ϴ��ҺҲע������ƿ��Ȼ��������ƿ��עˮ����Һ����̶���1��2CMʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�Ȼ��ҡ�ȡ�װƿ���ڴ˹������õ��������У���ƽ����Ͳ���ձ�����������500ml����ƿ����ͷ�ιܣ���ȱ�ٵ������У���ͷ�ιܡ�������������Ҫ���ǣ�ƽ����ƿ�ͷ�Һ©�������Դ��ǣ�A��D������������ͷ�ιܣ���2������ƿΪ����һ�����ʵ���Ũ����Һר������������ƿ�ϱ��������¶ȡ��������̶��ߣ�
��ѡ���٢ۢޣ���3������һ�����ʵ���Ũ����Һ�IJ��裺���㡢������ϡ�͡���ȴ����Һ�����ݡ�ҡ�ȡ�װƿ�ȣ�������ȷ��˳��Ϊ��BDAFEC��
���Դ��ǣ�BDAFEC����4������0.2mol/L NaOH��Һ500mL�� NaOH���������Ϊ��0.2mol/L��40g/mol��0.5L=4.0g��
���Դ��ǣ�4.0����5��Ũ����ϡ�Ͳ����������ȣ�ϡ�͵���ȷ����Ϊ����Ũ���������ձ��ڻ���ע��ˮ�У�ͬʱ�����ò��������裻
���Դ��ǣ���Ũ���������ձ��ڻ���ע��ˮ�У�ͬʱ�����ò��������裻��6��A������Ͳ��ȡŨ����ʱ��������Ͳ�Ŀ̶ȣ�������ȡ��Ũ�������ƫ�����ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���A��ѡ��
B������ƿδ���T����������Һ������Һ��������ʵ���������Ӱ�죬��ҺŨ�Ȳ��䣬��B��ѡ��
C��Ũ�������ձ���ϡ�ͺ�δ��ȴ������ת�Ƶ�����ƿ�У������ж��ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���C��ѡ��
D��������ƿת��ʱ��������Һ�彦�����������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Dѡ��
E��������ƿ�ж���ʱ��������ƿ�̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���E��ѡ��
F���ձ�δ����ϴ�ӣ��������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ���Fѡ��
G�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���Gѡ��
��ѡ��BFG��
�����㾫����������Ҫ����������һ�����ʵ���Ũ�ȵ���Һ�����֪ʶ�㣬��Ҫ�����������ʵ���Ũ����Һʱ�������ձ�������ˮ������ƿ�̶���1cm��2cm���ٸ��ý�Ͷ�ιܼ�ˮ���̶��߲�����ȷ�����⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪���������Ȼ�ѧ����ʽ��
H2(g)+ 1/2O2(g) ![]() H2O(l) ��H=��285.8 kJ��mol��1
H2O(l) ��H=��285.8 kJ��mol��1
C2H4(g)+ 3O2(g) ![]() 2CO2(g)+2H2O(l) ��H=��1411.0 kJ��mol��1
2CO2(g)+2H2O(l) ��H=��1411.0 kJ��mol��1
ʵ����H2��C2H4�Ļ�����干5 mol����ȫȼ������Һ̬ˮʱ����4242kJ,����������H2��C3H8���������____________��
��2����֪�����Ȼ�ѧ����ʽ:
��CH3COOH(l) + 2O2(g) ![]() 2CO2(g) + 2H2O(l)����H1=�D870.3 kJ��mol��1;
2CO2(g) + 2H2O(l)����H1=�D870.3 kJ��mol��1;
��C(s) + O2(g) ![]() CO2(g)����H2=�D393.5 kJ��mol��1;
CO2(g)����H2=�D393.5 kJ��mol��1;
��H2(g)+1/2 O2(g)![]() H2O(l)����H3=�D285.8 kJ��mol��1��
H2O(l)����H3=�D285.8 kJ��mol��1��
��Ӧ2C(s)+2H2(g)+O2(g)![]() CH3COOH(l)����HΪ______________kJ��mol��1��
CH3COOH(l)����HΪ______________kJ��mol��1��
��3����֪���з�Ӧ: ��SO2(g)+2OH��(aq)![]() SO32��(aq)+H2O(l)������H1
SO32��(aq)+H2O(l)������H1
��ClO�� (aq)+ SO32�� (aq)![]() SO42�� (aq)+Cl��(aq) ��H2
SO42�� (aq)+Cl��(aq) ��H2
��CaSO4(s)![]() Ca2+(aq)+ SO42�� (aq) ��H3 ��Ӧ
Ca2+(aq)+ SO42�� (aq) ��H3 ��Ӧ
SO2(g)+Ca2+(aq)+ClO��(aq)+2OH��(aq)![]() CaSO4(s)+H2O(l)+Cl��(aq)����H=_____________��
CaSO4(s)+H2O(l)+Cl��(aq)����H=_____________��
��4����֪:��̼��ȼ������H1= a kJ��mol��1
��S(s)+2K(s)![]() K2S(s)����H2=b kJ��mol��1
K2S(s)����H2=b kJ��mol��1
��2K(s)+N2(g)+3O2(g)![]() 2KNO3(s)����H3=c kJ��mol��1
2KNO3(s)����H3=c kJ��mol��1
�ڻ�ҩ��ը���Ȼ�ѧ����ʽΪ:
S(s)+2KNO3(s)+3C(s)![]() K2S(s)+N2(g)+3CO2(g)����H= ______________ kJ��mol��1
K2S(s)+N2(g)+3CO2(g)����H= ______________ kJ��mol��1
��5����2O2(g)+N2(g)![]() N2O4(l)��������H1 = a
N2O4(l)��������H1 = a
��N2(g)+2H2(g)![]() N2H4(l) ��H2=b ��O2(g)+2H2(g)
N2H4(l) ��H2=b ��O2(g)+2H2(g)![]() 2H2O(g) ��H3=c
2H2O(g) ��H3=c
��Ӧ2N2H4(l)+N2O4(l)![]() 3N2(g)+4H2O(g) ��H4=___________________��
3N2(g)+4H2O(g) ��H4=___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������и����������ʣ�
A��O2��O3 B�� ![]() C��
C��![]() C C��CH3CH2CH2CH3��CH4
C C��CH3CH2CH2CH3��CH4
D��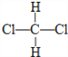 ��
�� 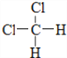 E��CH3CH2CH2CH3��
E��CH3CH2CH2CH3��
��1��________________����������Ϊͬλ�أ�(����ĸ����ͬ)
��2��________________���������ʻ�Ϊͬ�������壻
��3��________________��������������ͬϵ�
��4��________________�������ʻ�Ϊͬ���칹�壻
��5��_______________����������ͬһ���ʡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������ϣ��ڹ����³�ַ�Ӧ�����ò����У���CH3Cl����CH2Cl2����CHCl3����CCl4����HCl��������ȷ���� ( )
A���٢� B���ڢ�
C���٢ڢ��Ļ���� D���٢ڢۢܢ��Ļ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ��ϩ��һ�־�����̵��������ɱ�����õ����Ӽ�����ˮ��ϩ�ϳɾۺ���H��·����ͼ��ʾ��
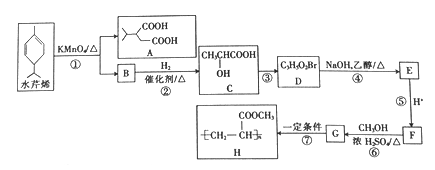
��֪��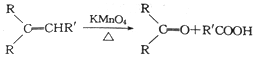
�ش��������⣺
��1��B�Ľṹ��ʽΪ_______________��C�Ļ�ѧ������_________________��
��2�����ķ�Ӧ�Լ��ͷ�Ӧ�����ֱ���_____________���÷�Ӧ�ķ�Ӧ������____________��
��3�����Ļ�ѧ����ʽΪ_____________������ŨH2SO4��������_______________��
��4��H�ķ���ʽΪ_________________��
��5��M��G��ͬ���칹�壬M����NaHCO3��Һ��Ӧ����CO2����M���ܵĽṹ��___________�֡�
��6�����ᡪ2��������![]() ������Ҫ���л������м��壬д����2-��-2-��ϩ��
������Ҫ���л������м��壬д����2-��-2-��ϩ��![]() ��Ϊԭ�ϣ������Լ���ѡ���Ʊ�����-2-������·�ߣ�____________��
��Ϊԭ�ϣ������Լ���ѡ���Ʊ�����-2-������·�ߣ�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڵ��ص�˵����ȷ����
A.��Դ�ĸ�����������
B.��ֱ����Դ�����������ǵ��ص�����
C.��ֱ����Դ���������ĵ缫�Ϸ�����ԭ��Ӧ
D.�����Ϸ�����ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��3molSO32��ǡ�ý�2molXO4�����ӻ�ԭ��SO32��������ΪSO42������XԪ���ڻ�ԭ�����еĻ��ϼ���
A. +1 B. +2 C. +3 D. +4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͬ���ʵ����ĸ��ֹ����Һ������������ͬ������Ҫԭ����
A�����Ӵ�С��ͬ B������������ͬ
C�����Ӽ���벻ͬ D���¶���ѹǿ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ƣ�NaClO2����һ�ָ�ЧƯ����ǿ��������ij��ȤС����NaClO3��ȡClO2���壬����ClO2�Ƶ�NaClO2��ʵ��װ�����£�

�ش��������⣺
��1����NaOH��������Լ20%��NaOH��Һ100g����Ҫ���������ձ�����������_____�� _____��
��2��ʵ����ʹNaClO3��������Ŀ���� _____��
��3��ΪʹClO2�����ܱ����ȡ�������գ�����ʱӦע�� _____��
��4��NaOH����ClO2β�����������ʵ���֮��Ϊ1��1�����������ӣ�һ��ΪClO2��������һ��Ϊ ___��
��5��B�з�Ӧ�����ӷ���ʽΪ ______��
��6��֤��NaClO2���������Եķ����ǣ���B����Һ���ȳ�ȥH2O2������ ______ ������ţ���ͬ���ữ���ټ��� ______ ������
��ϡHNO3 ��ϡH2SO4 ��K2SO3��Һ ��BaCl2��Һ ��FeCl2��Һ ��KSCN��Һ
��7��װ��C�������� ______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com