
����Ŀ�����ȷ�ұ���������Ŀ����к���Cr2O7��Al2O3������Fe2O3��������ȡ���������ᷨ�ͼ���ֹ��ա���ش�
I���ᷨ�������������ȡ��ȡҺͨ��������õ�����Cr����ʣ����Һ�мӼ���յõ�Al(OH)3��
(1)Ϊ��߿����Ľ�ȡ�ʣ��ɲ�ȡ�Ĵ�ʩ��_____(д������)��
(2)�����ʱ�������ĵ缫��ӦʽΪ______________��
II. ��������������£�
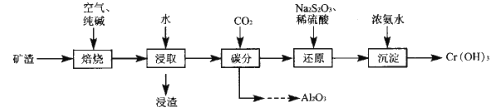
��֪�����������������ɷ�ΪNa2CrO4��NaAlO2��NaFeO2��
��![]() ��Zn2+������EDTA1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA��
��Zn2+������EDTA1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA��
(3)��������Ҫ�ɷ�ΪFe(OH)3��������ȡ��ʱ������Ӧ�����ӷ���ʽΪ_________��
(4)����ȡ����������Һ��Al�ĺ�������EDTA�ζ����ⶨ��
��ȡ20.00mL��ȡҺ����ƿ�У�����c1molL-1EDTA��ҺV1mL(�Թ���)��
�����������ᡢ�����������Ỻ����Һ����Һ�����ԣ����Ⱥ����PANָʾ����
����c2molL-1ZnSO4��Һ�ζ�����Һǡ�ó��Ϻ�ɫ�����ı�ҺV2mL��������ȡ����������Һ��Al�ĺ���Ϊ_________gL-1(�����ʽ����)��
(5)��̼����ʱͨ��CO2��ͨ��_____ (���������)�����ɵõ�������Al2O3��
(6)����ԭ��ʱ������Ҫ��Ӧ�����ӷ���ʽΪ__________��
(7)��������ʱ����c(Cr3+)��10-5molL-1ʱ��Ӧ������Һ��pH����Ϊ_________����Ksp[Cr(OH)3]=1.0��10-32��
���𰸡��ʵ���������Ũ�ȡ����߷�Ӧ�¶ȡ���С�������������ӽ�ȡʱ�䡢����(��������) Cr3++3e- = Cr ![]() +2H2O= Fe(OH)3��+OH-
+2H2O= Fe(OH)3��+OH- ![]() ���ˡ�ϴ�ӡ����� 8
���ˡ�ϴ�ӡ����� 8![]() +3
+3![]() +34H+=6
+34H+=6![]() +8Cr3++17H2O��4
+8Cr3++17H2O��4![]() +3
+3![]() +26H+=6
+26H+=6![]() +8Cr3++13H2O 5
+8Cr3++13H2O 5
��������
��.��Ͻ�ȡ���ʵ�Ӱ�����ط��������ݵ���ԭ���������
��.�������Ϣ�����ݹ������̷�����֪�������м��봿���ڿ����б�������Na2CrO4��NaAlO2��NaFeO2���ټ�ˮ��ȡ��������Ӧ![]() +2H2O=Fe(OH)3��+OH-������ΪFe(OH)3�����ȡҺ��ͨ��CO2��������Ӧ2H2O+NaAlO2+CO2=NaHCO3+Al(OH)3�������˺���Һ�к���Na2CrO4��������Һ�м���Na2S2O3��ϡ���ᣬNa2CrO4����ԭΪCr3+���ټ��백ˮ�����ɵõ�Cr(OH)3���ݴ˷������
+2H2O=Fe(OH)3��+OH-������ΪFe(OH)3�����ȡҺ��ͨ��CO2��������Ӧ2H2O+NaAlO2+CO2=NaHCO3+Al(OH)3�������˺���Һ�к���Na2CrO4��������Һ�м���Na2S2O3��ϡ���ᣬNa2CrO4����ԭΪCr3+���ټ��백ˮ�����ɵõ�Cr(OH)3���ݴ˷������
��.(1)���ʱ���ɲ����ʵ���������Ũ�ȡ����߷�Ӧ�¶ȡ���С�������������ӽ�ȡʱ�䡢����ȷ�����߿����Ľ�ȡ�ʣ��ʴ�Ϊ���ʵ���������Ũ�ȡ����߷�Ӧ�¶ȡ���С�������������ӽ�ȡʱ�䡢����(��������)��
(2)�����ʱ��C3+�������õ���������Cr���缫��ӦʽΪCr3++3e- =Cr���ʴ�Ϊ��Cr3++3e- =Cr��
��.(3)��������������֪����ˮ��ȡ��������Ӧ![]() +2H2O=Fe(OH)3��+OH-������ΪFe(OH)3���ʴ�Ϊ��
+2H2O=Fe(OH)3��+OH-������ΪFe(OH)3���ʴ�Ϊ��![]() +2H2O=Fe(OH)3��+OH-��
+2H2O=Fe(OH)3��+OH-��
(4)��֪![]() ��Zn2+������EDTA��1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA����
��Zn2+������EDTA��1��1��ϳ������ӣ�Zn2+����PAN1��1��ϳ��Ϻ�ɫ�����ҽ����������EDTA����![]() ���ĵ�EDTA�����ʵ���Ϊ(c1V1-c2V2)��10-3mol����Al�����ʵ���Ϊ(c1V1-c2V2)��10-3mol��������Ϊ27g/mol��(c1V1-c2V2)��10-3mol=27(c1V1-c2V2)��10-3g������Һ��Al�ĺ���Ϊ
���ĵ�EDTA�����ʵ���Ϊ(c1V1-c2V2)��10-3mol����Al�����ʵ���Ϊ(c1V1-c2V2)��10-3mol��������Ϊ27g/mol��(c1V1-c2V2)��10-3mol=27(c1V1-c2V2)��10-3g������Һ��Al�ĺ���Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(5)��̼��/span>��ʱ�����ȡҺ��ͨ��CO2��������Ӧ2H2O+NaAlO2+CO2=NaHCO3+Al(OH)3�������˵õ�Al(OH)3������������ϴ�Ӻ���ȿ�ֱ�ӷֽ�õ�������Al2O3���ʴ�Ϊ�����ˡ�ϴ�ӡ����ȣ�
(6)���˺���Һ�к���Na2CrO4��������Һ�м���Na2S2O3��ϡ���ᣬNa2CrO4����ԭΪCr3+������������ԭ��Ӧ��ʧ�����غ���ɿɵã���Ӧ�����ӷ���ʽΪ8![]() +3
+3![]() +34H+=6
+34H+=6![]() +8Cr3++17H2O��4
+8Cr3++17H2O��4![]() +3
+3![]() +26H+=6
+26H+=6![]() +8Cr3++13H2O���ʴ�Ϊ��8
+8Cr3++13H2O���ʴ�Ϊ��8![]() +3
+3![]() +34H+=6
+34H+=6![]() +8Cr3++17H2O��4
+8Cr3++17H2O��4![]() +3
+3![]() +26H+=6
+26H+=6![]() +8Cr3++13H2O��
+8Cr3++13H2O��
(7)���ݳ����ܽ�ƽ��Cr(OH)3![]() Cr3++3OH-�ɵã�Ksp[Cr(OH)3]=c(Cr3+)��c3(OH-)����c(Cr3+)=10-5molL-1ʱ��
Cr3++3OH-�ɵã�Ksp[Cr(OH)3]=c(Cr3+)��c3(OH-)����c(Cr3+)=10-5molL-1ʱ�� ����pOH=-lg[c(OH-)]=9��pH=14-pOH=5������Ӧ������Һ��pH����Ϊ5���ʴ�Ϊ��5��
����pOH=-lg[c(OH-)]=9��pH=14-pOH=5������Ӧ������Һ��pH����Ϊ5���ʴ�Ϊ��5��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�á�
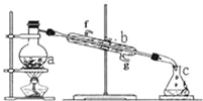
��1��д���������������ƣ�a��__b��__��
��2��ʵ������У���Ҫͨ��ˮ��ͼ�еĽ�ˮ������__������ͼ����ĸ����
��3��������װ�÷������Ȼ�̼�;ƾ��Ļ�����ȱ�ٵ���Ʒ��__��
��4������װ��������ˮ��ʵ��ʱa�г�������������ˮ�⣬�����������__���������Ƿ�ֹ���С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л���a( )��b(
)��b(![]() )��c(
)��c( )��˵���������
)��˵���������
A. a��b��c��Ϊͬ���칹��
B. a��c����ʹ���Ը��������Һ��ɫ
C. a��c������������Ӧ�������������ʵ���֮����4��3
D. a��b��c��һ�ȴ���ֱ���4�֡�1�֡�2��(�����������칹)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ���л�����ԭ�ϣ����������Ʊ�ϩ���������ľ��ü�ֵ��������塣�ش��������⡣
��1����֪��I.2C3H8(g)+O2(g)=2C3H6(g)+2H2O(g) ��H=-238kJ��mol-1
II.2H2(g)+O2(g)=2H2O(g) ��H=-484kJ��mol-1
����������Ʊ�ϩ��ӦC3H8(g)![]() C3H6(g)+H2(g)����HΪ___��
C3H6(g)+H2(g)����HΪ___��
��2����ͼΪ����ֱ�����ⷨ�б���ͱ�ϩ��ƽ������������¶ȡ�ѹǿ�Ĺ�ϵ(ͼ�е�ѹǿ�ֱ�Ϊ104Pa��105Pa)��
104Paʱ��ͼ�б�ʾ��ϩ��������__(�������������������������)��
��3��һ���¶��£�������ܱ������г���1molC3H8����ʼѹǿΪpkPa���������������Ʊ�ϩ��Ӧ��
�����������˵�����������Ʊ�ϩ��Ӧ�ﵽƽ��״̬����__(����ĸ)��
A.�÷�Ӧ���ʱ�(��H)���ֲ���
B.����ƽ��Ħ���������ֲ���
C.�����ܶȱ��ֲ���
D.C3H8�ֽ�������C3H6�����������
����ʹ��ϩ��ƽ�������ߣ�Ӧ��ȡ�Ĵ�ʩ��__�����ţ���
A.�����¶� B.�����¶� C.����ѹǿ D.����ѹǿ
��Ϊ�ṩ��Ӧ������������ѹʱ����ԭ�����в���ˮ��������������ⷴӦ��K__(���������С�����䡱)
��4�����������Ʊ�ϩ��Ӧ�����У�C3H8��������������뷴Ӧʱ��Ĺ�ϵ��ͼa��ʾ�����¶��¸÷�Ӧ��ƽ�ⳣ��Kp=__kPa(�ú���ĸp�Ĵ���ʽ��ʾ��Kp���÷�Ӧ��ϵ���������ʵķ�ѹ��ʾ��ƽ�ⳣ����ƽ���ѹ=��ѹ�����ʵ�������)��
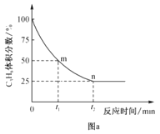
��5������CO2���������ԣ������˱������������Ʊ�ϩ���¹��ա��ù��տɲ��ø���������Ϊ�������䷴Ӧ������ͼ��
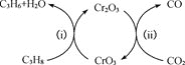
ͼ�д���Ϊ__���ù��տ�����Ч������������Ļ�̿��ά�ִ������ԣ�ԭ����__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
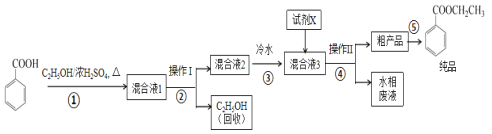
����Ŀ����������������Ҫ�ľ�ϸ�����Լ�������������ˮ����ʳ���㾫��ʵ�����Ʊ�������ͼ��
�Լ�����������±���
������ | �Ҵ� | ���������� | |
������״ | ��ɫ��״���� | ��ɫҺ�� | ��ɫ��Һ�� |
�е�/�� | 249.0 | 78.0 | 212.6 |
��Է����� | 122 | 46 | 150 |
�ܽ��� | ����ˮ���������Ҵ������ѵ��л��ܼ� | ��ˮ����Ȼ��� | ��������ˮ��������ˮ���������Ҵ������� |
�ش��������⣺
(1)Ϊ���ԭ�ϱ�����Ĵ��ȣ��ɲ��õĴ�������Ϊ__��
(2)����ٵ�װ����ͼ��ʾ(���Ⱥͼг�װ������ȥ)����һС������������B�п��������״�������ˮ��(��ˮ����ͭ���Ҵ�������Һ)��������B�У�������C�м���12.2g������ı����ᾧ�壬30mL��ˮ�Ҵ�(Լ0.5mol)��3mLŨ���ᣬ�����ʯ���������У�������Ӧ1.5��2h������A��������__��
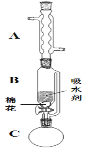
(3)���ŷ�Ӧ���У���Ӧ��ϵ��ˮ�ֲ��ϱ���Ч���룬����B����ˮ��������Ϊ__��
(4)��Ӧ������C�л��Һ���з����ᴿ������I��_������II���õIJ������������ձ����__��
(5)��Ӧ����������н���ӦҺ������ˮ��Ŀ�ij����ܽ��Ҵ��⣬����__�������Լ�XΪ___(��д��ѧʽ)��
(6)���յõ����﴿Ʒ10.0g��ʵ�����Ϊ__%(������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��Ϊ�л������A����������һ������ʯ�ͻ�����չˮƽ�ı�־�����ʣ�����֮��������ת����ϵ��
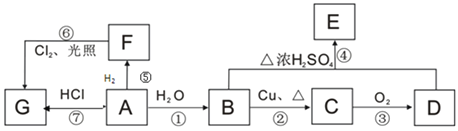
��֪��![]() ����ش��������⣺
����ش��������⣺
(1)B��D�й����ŵ����ƣ�B____________��D________________��
(2)ָ�����б�Ŷ�Ӧ��Ӧ�ķ�Ӧ���ͣ���____________����_____________��
(3)��F��ͬϵ��������л���Ŀռ乹��Ϊ___________������ʽΪ____________��
(4)д����E������ͬ�����ţ�����E��������ͬ���칹��Ľṹ��ʽ��_________��
(5)д�����б�Ŷ�Ӧ��Ӧ�Ļ�ѧ��Ӧ����ʽ��
��_______________________��
��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������10mL pH=3�Ĵ�����Һ�м���ˮϡ�ͺ�����˵����ȷ����
A. ��Һ�е������ӵ���Ŀ����
B. ��Һ��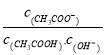 ����
����
C. ����ĵ���̶�����C��H+��������
D. �ټ���10mlpH=11��NaOH��Һ�����ҺpH=7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����û�ѧ��Ӧԭ�����й�֪ʶ�ش��������⣺
(1)�������������ȼ�յ��Ȼ�ѧ����ʽΪSi(s)+O2(g)=SiO2(s)��H=-989.2 kJ��mol-1���йؼ����������±���
��ѧ�� | Si-O | O=O | Si-Si |
����kJ��mol-1 | X | 498.8 | 176 |
��X��ֵΪ_________��
(2)����N2O5�����η����ķֽⷴӦΪ��N2O5![]() N2O3+O2����N2O3
N2O3+O2����N2O3![]() N2O+O2����1 L�ܱ������г���4 mol N2O5�����ȵ�t �棬�ﵽƽ��״̬��O2��ƽ��Ũ��Ϊ4.5 mol/L��N2O3��ƽ��Ũ��Ϊ1.7 mol/L����t��ʱ��Ӧ�ٵ�ƽ�ⳣ��Ϊ_________��
N2O+O2����1 L�ܱ������г���4 mol N2O5�����ȵ�t �棬�ﵽƽ��״̬��O2��ƽ��Ũ��Ϊ4.5 mol/L��N2O3��ƽ��Ũ��Ϊ1.7 mol/L����t��ʱ��Ӧ�ٵ�ƽ�ⳣ��Ϊ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.4molX�����0.6molY��������2L�ܱ������У�ʹ���Ƿ������·�Ӧ��4X(g)+5Y(g) ![]() nZ(g)+6W(g)��2minĩ������0.3molW������֪��Z��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.05mol/(L��min)���Լ���
nZ(g)+6W(g)��2minĩ������0.3molW������֪��Z��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.05mol/(L��min)���Լ���
��1��ǰ2min����W��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ_______________��
��2��2minĩʱY��Ũ��Ϊ_____________________________��
��3����ѧ��Ӧ����ʽ��n=_____________________________��
��4��2minĩ���ָ�����Ӧǰ�¶ȣ���ϵ��ѹǿ�Ƿ�Ӧǰѹǿ��__________����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com