
��ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���
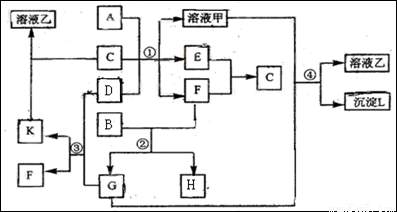
��1��A��B��C��D���������ʷֱ�Ϊ �� �� �� ���ѧʽ����
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
 ��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ���
�� ���ѧʽ����
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ���
�� ���ѧʽ����
 ��4����Ӧ�۲�����K�Ļ�ѧʽΪ
��
��4����Ӧ�۲�����K�Ļ�ѧʽΪ
��
 ��5����Ӧ�ܵ����ӷ���ʽΪ
��
��5����Ӧ�ܵ����ӷ���ʽΪ
��
��1��Al C Na2O2
(2) 2H2O +Na2O2 =4NaOH+O2�� 2Al+ 2NaOH+2H2O=2NaAlO2+3H2��
 (3) CO2 CO (4)
Na2CO3 (5) 2AlO2-+CO2+3H2O=2Al(OH)3��+CO32-
(3) CO2 CO (4)
Na2CO3 (5) 2AlO2-+CO2+3H2O=2Al(OH)3��+CO32-
��������D�ǵ���ɫ�Ĺ��廯�����DZ����ͻ�ƿڣ�������ѧ��ѧ֪ʶ��������Na2O2������ˮ��CO2��Ӧ��������Ŀ��һ��ϢC�dz�������ɫ��ζҺ��˵��C��ˮ����GΪCO2��������K������ˮ��˵��KΪNa2CO3 ��FΪO2; �����Ϣ��ɫ����B��������F����Ӧ�õ�G��CO2����˵��BΪC��̼��������F��O2����E��Ӧ����C(ˮ)��֪EΪ�������ٸ��ݽ���A�������Һ��Ӧ�����������ɵõ�AΪAl.


 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2009?��������ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԣ���
��2009?��������ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

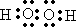
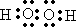
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���
![]()
![]() ��1��A��B��C��D���������ʷֱ�Ϊ���� ������ �������� �������� ���ѧʽ����
��1��A��B��C��D���������ʷֱ�Ϊ���� ������ �������� �������� ���ѧʽ����
![]() ��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�������� ��
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�������� ��
![]() ��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ������� ������ ���ѧʽ����
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ������� ������ ���ѧʽ����
![]() ��4����Ӧ�۲�����K�Ļ�ѧʽΪ���� ����������������������������
��4����Ӧ�۲�����K�Ļ�ѧʽΪ���� ����������������������������
![]() ��5����Ӧ�ܵ����ӷ���ʽΪ���� ��������������������������������������
��5����Ӧ�ܵ����ӷ���ʽΪ���� ��������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���
![]()
![]()
![]()
![]()

��1��A��B��C��D���������ʷֱ�Ϊ �� �� �� ���ѧʽ����
![]() ��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ��� �� ���ѧʽ����
��4����Ӧ�۲�����K�Ļ�ѧʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��09���ľ�����ͼ��ʾ�й����ʣ����ɶ�����Ԫ���γɣ�֮���ת����ϵ������AΪ�����Ľ������ʣ�BΪ�ǽ������ʣ�һ���Ǻ�ɫ��ĩ����C�dz�������ɫ��ζҺ�壬D�ǵ���ɫ�Ĺ��廯�������Ӧ����ͼ����ʡ�ԡ���

��1��A��B��C��D���������ʷֱ�Ϊ �� �� �� ���ѧʽ����
��2����Ӧ���е�C��D���������÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3����Ӧ���У���B��F���ʵ���֮��Ϊ4��3��G��H�ֱ��� �� ���ѧʽ����
��4����Ӧ�۲�����K�Ļ�ѧʽΪ ��
��5����Ӧ�ܵ����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com