
��16�֣�
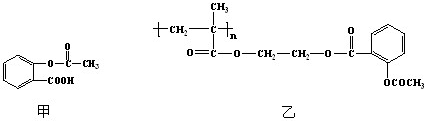
��A���Ҵ�Ϊ�л�����ԭ�ϣ��ϳ����ϼ�����ͼ���£�
��֪��R��Br+NaCN��R��CN+NaBr��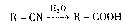
�����к���A��A�ܷ���������Ӧ�����ײⶨ��A����Է�������Ϊ106��5��3g A��ȫ
ȼ��ʱ������15��4g CO����2��7g HO��
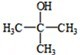
��1��A�ķ���ʽΪ ��A����������Ӧ�Ļ�ѧ����ʽΪ ��
��2��д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��������Ӧ������ȡ����Ӧ���ǣ� ��
��4����������������E��ͬ���칹���� �֣�
a���ܷ���������Ӧ b������Cu c������ˮ��
д������2�ֽṹ��ʽ ��
��5��д����Ӧ�ݵĻ�ѧ����ʽ ��
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���������������ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��16�֣�
��A���Ҵ�Ϊ�л�����ԭ�ϣ��ϳ����ϼ�����ͼ���£�
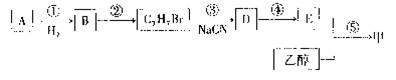
��֪��R��Br+NaCN��R��CN+NaBr��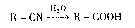
�����к���A��A�ܷ���������Ӧ�����ײⶨ��A����Է�������Ϊ106��5��3g A��ȫ
ȼ��ʱ������15��4g CO����2��7g HO��
��1��A�ķ���ʽΪ ��A����������Ӧ�Ļ�ѧ����ʽΪ ��
��2��д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��������Ӧ������ȡ����Ӧ���ǣ� ��
��4����������������E��ͬ���칹���� �֣�
a���ܷ���������Ӧ b������Cu c������ˮ��
д������2�ֽṹ��ʽ ��
��5��д����Ӧ�ݵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A���Ҵ�Ϊ�л�����ԭ�ϣ��ϳ����ϼ�����ͼ���£�
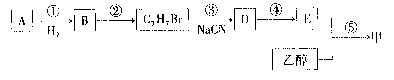
��֪��R��Br+NaCN��R��CN+NaBr��![]()
�����к���A��A�ܷ���������Ӧ�����ײⶨ��A����Է�������Ϊ106��5��3g A��ȫ
ȼ��ʱ������15��4g CO����2��7g HO��
��1��A�ķ���ʽΪ ��A����������Ӧ�Ļ�ѧ����ʽΪ ��
��2��д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��������Ӧ������ȡ����Ӧ���ǣ� ��
��4����������������E��ͬ���칹���� �֣�
a���ܷ���������Ӧ b������Cu c������ˮ��
д������2�ֽṹ��ʽ ��
��5��д����Ӧ�ݵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣�
��A���Ҵ�Ϊ�л�����ԭ�ϣ��ϳ����ϼ�����ͼ���£�
��֪��R��Br+NaCN��R��CN+NaBr��
�����к���A��A�ܷ���������Ӧ�����ײⶨ��A����Է�������Ϊ106��5��3g A��ȫ
ȼ��ʱ������15��4g CO����2��7g HO��
��1��A�ķ���ʽΪ ��A����������Ӧ�Ļ�ѧ����ʽΪ ��
��2��д����Ӧ�ڵĻ�ѧ����ʽ ��
��3��������Ӧ������ȡ����Ӧ���ǣ� ��
��4����������������E��ͬ���칹���� �֣�
a���ܷ���������Ӧ b������Cu c������ˮ��
д������2�ֽṹ��ʽ ��
��5��д����Ӧ�ݵĻ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com