

���� ��ij������1.65g A��������Ԫ�أ����ڹ������ᣬ����ʽ��Ϊ44������B��BӦΪCO2��A�к���̼Ԫ�ء���Ԫ�أ����˵õ�����0.06g�IJ���C��������Ϊ53.2%��������ϡ�����ҺD��ȡ����D��Һ������С���KSCN��Ѫ��ɫ����D�к���Fe3+����A�к���FeԪ�أ���4������C����Ԫ����ͬ�Ļ�����E��ʽ����44������ѧ�����������ڹ�ҵ�ƹ�ķ�Ӧ�����з��֣���ҵ�Ʊ�Si��ӦΪ��2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO��������֪CΪSiO2��EΪSiO��SiO2��Ԫ����������Ϊ53.2%����״���£�CO2�����0.224L�����ʵ���Ϊ0.01mol����A��n��CO32-��=n��CO2��=0.01mol��m��SiO2��=0.6g����n��SiO2��=$\frac{0.6g}{60g/mol}$=0.01mol��ȥ��̼��������������ʣ��������Ϊ1.68g-0.01mol��60g/mol-0.6g=0.48g����û���������ۻ��ţ����ݵ���غ��֪��n��Fe3+��=$\frac{0.01mol��2}{3}$����FeԪ������Ϊ$\frac{0.01mol��2}{3}$��56g/mol=0.373g��0.48g����0.48gΪFeԪ�ء�OԪ����������0.48g��FeΪxmol��Oԭ��Ϊymol���ɵ���غ㣺3x=2y+0.01��2���ɶ��������ɵã�56x+16y=0.48���������x=0.008��y=0.002��Fe2O3Ϊ$\frac{0.002}{3}$mol��Fe2��CO3��3Ϊ$\frac{0.01}{3}$mol����Fe2O3��Fe2��CO3��3��SiO2�����ʵ���֮��Ϊ$\frac{0.002}{3}$mol��$\frac{0.01}{3}$mol��0.01mol=1��5��5��A�Ļ�ѧʽΪFe2O3•5Fe2��CO3��3•15SiO2���ݴ˽��
��� �⣺��ij������1.65g A��������Ԫ�أ����ڹ������ᣬ����ʽ��Ϊ44������B��BӦΪCO2��A�к���̼Ԫ�ء���Ԫ�أ����˵õ�����0.06g�IJ���C��������Ϊ53.2%��������ϡ�����ҺD��ȡ����D��Һ������С���KSCN��Ѫ��ɫ����D�к���Fe3+����A�к���FeԪ�أ���4������C����Ԫ����ͬ�Ļ�����E��ʽ����44������ѧ�����������ڹ�ҵ�ƹ�ķ�Ӧ�����з��֣���ҵ�Ʊ�Si��ӦΪ��2C+SiO2$\frac{\underline{\;����\;}}{\;}$Si+2CO��������֪CΪSiO2��EΪSiO��SiO2��Ԫ����������Ϊ53.2%����״���£�CO2�����0.224L�����ʵ���Ϊ0.01mol����A��n��CO32-��=n��CO2��=0.01mol��m��SiO2��=0.6g����n��SiO2��=$\frac{0.6g}{60g/mol}$=0.01mol��ȥ��̼��������������ʣ��������Ϊ1.68g-0.01mol��60g/mol-0.6g=0.48g����û���������ۻ��ţ����ݵ���غ��֪��n��Fe3+��=$\frac{0.01mol��2}{3}$����FeԪ������Ϊ$\frac{0.01mol��2}{3}$��56g/mol=0.373g��0.48g����0.48gΪFeԪ�ء�OԪ����������0.48g��FeΪxmol��Oԭ��Ϊymol���ɵ���غ㣺3x=2y+0.01��2���ɶ��������ɵã�56x+16y=0.48���������x=0.008��y=0.002��Fe2O3Ϊ$\frac{0.002}{3}$mol��Fe2��CO3��3Ϊ$\frac{0.01}{3}$mol����Fe2O3��Fe2��CO3��3��SiO2�����ʵ���֮��Ϊ$\frac{0.002}{3}$mol��$\frac{0.01}{3}$mol��0.01mol=1��5��5��A�Ļ�ѧʽΪFe2O3•5Fe2��CO3��3•15SiO2��
��1��BΪCO2������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��������������֪��A�Ļ�ѧʽΪFe2O3•5Fe2��CO3��3•15SiO2��
�ʴ�Ϊ��Fe2O3•5Fe2��CO3��3•15SiO2��
��3��D�к���Fe3+�������������������Һ�����ɺ��ɫ���������ӷ���ʽΪ��Fe3++3OH-=Fe��OH��3����
�ʴ�Ϊ��Fe3++3OH-=Fe��OH��3����
��4��EΪSiO��������E�ķ�Ӧ���������뻹ԭ����Ϊͬһ���ʣ�Ӧ��Si��SiO2��Ӧ����Ӧ����ʽΪ��Si+SiO2$\frac{\underline{\;����\;}}{\;}$2SiO���ʴ�Ϊ��Si+SiO2$\frac{\underline{\;����\;}}{\;}$2SiO��
���� ���⿼�������ƶϣ����ڼ������ƶϣ����ؿ���ѧ���ķ���������������Ҫѧ����������Ԫ�ػ�����֪ʶ���ѶȽϴ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6.2 g���ף�P4��������P-P������ĿΪ0.15NA�������ӽṹ �� �� | |
| B�� | ���³�ѹ�£�16 g 14CH4����������Ϊ8NA | |
| C�� | 117 g�Ȼ��ƹ����к���2NA���Ȼ��Ʒ��� | |
| D�� | ��5NA�����ӵİ����У���ԭ����ĿΪ1.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
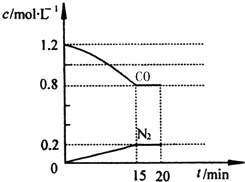
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.265mol•L-1 | B�� | 0.525mol•L-1 | C�� | 0.21mol•L-1 | D�� | 0.42mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ⱥ��ʳ��ˮʱ�������ĵ缫��ӦʽΪ��2Cl--2e-�TCl2�� | |
| B�� | �ڶƼ��ϵ��ͭʱ���öƼ����������缫��ӦʽΪ��Cu2++2e-�TCu | |
| C�� | ��ͭ����ʱ�����Դ�����������Ǵ�ͭ���缫��ӦʽΪ��Cu-2e-�TCu2+ | |
| D�� | ��������������ʴʱ��������ӦʽΪ��O2+2H2O+4e-�T4OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
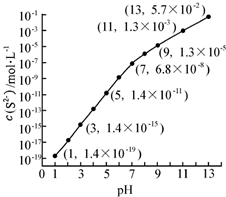 ��1��25�棬��0.10mol•L-1H2S��Һ�У�ͨ��HCl��������NaOH�����Ե�����ҺpH����ҺpH��c��S2-����ϵ��ͼ��������Һ����ı仯��H2S�Ļӷ�����
��1��25�棬��0.10mol•L-1H2S��Һ�У�ͨ��HCl��������NaOH�����Ե�����ҺpH����ҺpH��c��S2-����ϵ��ͼ��������Һ����ı仯��H2S�Ļӷ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C60��Ӣ����������ѧ�ҹ�ͬ���ֵģ������˹��ʿƼ���������Ҫ�� | |
| B�� | �Ž��з���ǰ�˹����Ļ����Ϸ�����Ԫ�������ɣ�������ѧ�о���Ҫ�̳���Ҫ���� | |
| C�� | �ƶ��Ͳ��ն������ۻ�ѧ����Ĺ���ŵ������ѧ������ζ�Ż�ѧ�ѳ�Ϊ�������о�Ϊ����ѧ�� | |
| D�� | ά��������ϳ������أ�ͻ�����������л���Ľ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com