|
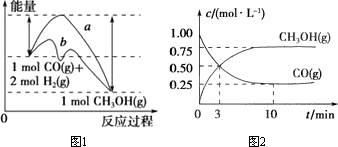
��ҵ����CO������ȼ�ϼ״���һ�������·�����Ӧ��CO(g)��2H2(g)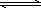 CH3OH(g)��ͼ1��ʾ��Ӧ�����������ı仯�����ͼ2��ʾһ���¶��£���̶����Ϊ2 L�������м���4 mol��H2��һ������CO��CO(g)��CH3OH(g)�����ʵ��� CH3OH(g)��ͼ1��ʾ��Ӧ�����������ı仯�����ͼ2��ʾһ���¶��£���̶����Ϊ2 L�������м���4 mol��H2��һ������CO��CO(g)��CH3OH(g)�����ʵ���
��ش��������⣺
(1)��ͼ1�У�����________(�a����b��)��ʾ��Ӧʹ���˴������÷�Ӧ����________(����ȡ����ȡ�)��Ӧ��
(2)����ͼ2�ж�����˵������ȷ����________(�����)��
a����ʼ�����CO�����ʵ���Ϊ2 mol
b������CO��Ũ�ȣ�CO��ת��������
c��������ѹǿ�㶨ʱ��˵����Ӧ�Ѵ�ƽ��״̬
d�������¶Ⱥ��ܱ��������ݻ����䣬�ٳ���1 mol��CO��2 mol��H2���ٴδﵽƽ��ʱ��n(CH3OH)/n(CO)��ֵ���С��
(3)�ӷ�Ӧ��ʼ���ﵽƽ�⣬��Ӧ����v(H2)��________�����¶���CO(g)��2H2(g)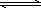 CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ________�������������������䣬����Ӧ��ϵ���£���÷�Ӧ�Ļ�ѧƽ�ⳣ����________(�������С�����䡱)�� CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ________�������������������䣬����Ӧ��ϵ���£���÷�Ӧ�Ļ�ѧƽ�ⳣ����________(�������С�����䡱)��
(4)��֪CH3OH(g)��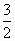 O2(g) O2(g) CO2(g)��2H2O(g)����H����192.9 kJ��mol��1��H2O(l) CO2(g)��2H2O(g)����H����192.9 kJ��mol��1��H2O(l) H2O(g)����H����44 kJ��mol��1����д��32 g��CH3OH(g)��ȫȼ������CO2��Һ̬ˮ���Ȼ�ѧ����ʽ��________�� H2O(g)����H����44 kJ��mol��1����д��32 g��CH3OH(g)��ȫȼ������CO2��Һ̬ˮ���Ȼ�ѧ����ʽ��________��
| 
 ������������ϵ�д�
������������ϵ�д�