
������������C3H6�ϳ��л��߷���E��C6H14������ͼ����ش��������⣺
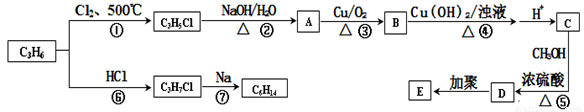
(1)��〜��������ȡ����Ӧ����_______________��
(2)C6H14�˴Ź�����ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ��_________________,
д��E�Ľṹ��ʽ��___________________��
(3)д��B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ��________________________��
(4)D��ͬ���칹��ܶ࣬��������������ͬ���칹����___�֣�������ԭ�Ӻ˴Ź����������ٵĽṹ��ʽΪ_______��
�ٺ�̼̼˫�� ����ˮ�� ���ܷ���������Ӧ
(5)��������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ�����(���Լ���ѡ����д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ���л�����д�ṹ��ʽ��:
________________________________��________________________________________��
��1���٢ڢݣ�2�֣� ��2����2�֣���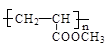 ��2�֣�
��2�֣�
��3��CH2��CHCHO +
2Cu(OH)2+NaOH CH2��CHCOONa + Cu2O��+3H2O ��2�֣�
CH2��CHCOONa + Cu2O��+3H2O ��2�֣�
��4�� 3��2�֣���  ��1�֣�
��1�֣�
��5��CH3CH2OH  CH2��CH2�� + H2O
��2�֣�
CH2��CH2�� + H2O
��2�֣�
3BrCH2CH2Br +6 Na 
 + 6NaBr ��2�֣�
+ 6NaBr ��2�֣�
��������
���������C3H6Ӧ���DZ�ϩ��������Ӧ��ȡ����Ӧ���õ�CH2=CHCH2Cl����ˮ���CH2=CHCH2OH����������CH2=CHCHO���ܼ���������CH2=CHCOOH���ݷ���������Ӧ��CH2=CHCOOCH3���ټӾ۵�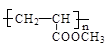 ���ӳɵ�CH3CHClCH3���߿ɵ�(CH3)2CHCH(CH3)2��D��ͬ���칹���ܷ���������Ӧ��һ���Ǽ�������������HCOOCH2CH=CH2��HCOOCH=CHCH3��
���ӳɵ�CH3CHClCH3���߿ɵ�(CH3)2CHCH(CH3)2��D��ͬ���칹���ܷ���������Ӧ��һ���Ǽ�������������HCOOCH2CH=CH2��HCOOCH=CHCH3��
���㣺�����л�����������ʡ�ͬ���칹��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ��ɽ�и�����һ��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
������������C3H6�ϳ��л��߷���E����C6H14������ͼ����ش��������⣺

��1����〜��������ȡ����Ӧ����______��
��2��C6H14�˴Ź�����ֻ�����ַ壬��C6H14�Ľṹ��ʽΪ�� ___________��д��E�Ľṹ��ʽ�� __________��
��3��д��B������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ�� __________��
��4��D��ͬ���칹��ܶ࣬��������������ͬ���칹����___ _�֣�������ԭ�Ӻ˴Ź����������ٵĽṹ��ʽΪ__________��
�ٺ�̼̼˫�� ����ˮ�� ���ܷ���������Ӧ
��5����������ѧ֪ʶ����ͼ�������Ϣ�����Ҵ�Ϊ��Ҫԭ��ͨ���������ܺϳɻ����� (���Լ���ѡ����д����һ���͵�������ѧ��Ӧ�Ļ�ѧ����ʽ���л�����д�ṹ��ʽ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com