
ÓŅĶ¼ĪŖŹµŃéŹŅijÅØĮņĖįĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż£¬
|
£Ø1£©øĆÅØĮņĖįµÄĪļÖŹµÄĮæÅضČĪŖ ”£
£Ø2£©Ź¹ÓĆČŻĮæĘæĒ°±ŲŠė½ųŠŠµÄŅ»²½²Ł×÷ŹĒ___________
£Ø3£©Ä³Ń§ÉśÓūÓĆÉĻŹöÅØĮņĖįŗĶÕōĮóĖ®ÅäÖĘ500 ml ĪļÖŹµÄĮæ
ÅضČĪŖ1.0 mol”¤L-1Ļ”ĮņĖį”£
¢ŁøĆѧɜŠčŅŖĮæČ” ml ÉĻŹöÅØĮņĖį½ųŠŠÅäÖĘ”£
¢ŚČŻĮæĘæÉĻŠč±źÓŠŅŌĻĀĪåĻīÖŠµÄ
AĪĀ¶Č BÅØ¶Č CČŻĮæ DŃ¹Ēæ EæĢ¶ČĻß
¢ŪŌŚÅäÖĘ¹ż³ĢÖŠ£¬ĻĀĮŠŹµŃé²Ł×÷¶ŌĖłÅäÖʵÄĻ”ĮņĖįµÄĪļÖŹµÄĮæÅضČÓŠŗĪÓ°Ļģ£æ
£ØŌŚŗóĆęŗįĻßÉĻ”°Ę«øß”±”¢”°Ę«µĶ”±”¢”°ĪŽÓ°Ļģ”±£©”£
I”¢ÓĆĮæĶ²ĮæČ”ÅØĮņĖįŹ±ø©ŹÓ°¼ŅŗĆę””””””””
II”¢¶ØČŻŗó¾Õńµ“”¢Ņ”ŌČ”¢¾²ÖĆ£¬·¢ĻÖŅŗĆęĻĀ½µ£¬ŌŁ¼ÓŹŹĮæµÄÕōĮóĖ®””””””””””””
¢ó”¢¶ØČŻŹ±ŃöŹÓŹÓæĢ¶ČĻß
¢ō”¢×ŖŅĘŹ±ČŻĮæĘæÖŠÓŠÉŁĮæÕōĮóĖ®
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
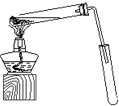 ij»ÆѧŹµŃ銔×éÓĆÓŅĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬²¢¼ģŃéŅŅ Ėį ŅŅ õ„ÖŠŹĒ·ńŗ¬ÓŠŅŅĖįŌÓÖŹ£ØĢś¼ÜĢØ”¢¼Š×ÓµČÖ§³ÅŅĒĘ÷Ź”ĀŌ£©£®ŅŃÖŖŅŅĖįŅŅõ„µÄ·ŠµćĪŖ77.1”ę£¬ŅŅ“¼·ŠµćĪŖ78.4”ę£¬ŅŅĖįµÄ·ŠµćĪŖ118”ę£®Ēėøł¾ŻŅŖĒóĢīæÕ£ŗ
ij»ÆѧŹµŃ銔×éÓĆÓŅĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„£¬²¢¼ģŃéŅŅ Ėį ŅŅ õ„ÖŠŹĒ·ńŗ¬ÓŠŅŅĖįŌÓÖŹ£ØĢś¼ÜĢØ”¢¼Š×ÓµČÖ§³ÅŅĒĘ÷Ź”ĀŌ£©£®ŅŃÖŖŅŅĖįŅŅõ„µÄ·ŠµćĪŖ77.1”ę£¬ŅŅ“¼·ŠµćĪŖ78.4”ę£¬ŅŅĖįµÄ·ŠµćĪŖ118”ę£®Ēėøł¾ŻŅŖĒóĢīæÕ£ŗ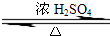 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O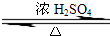 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ijĶ¬Ń§ĪŖÖĘČ”ŅŅĖįŅŅõ„£¬ŌŚŹŌ¹ÜAÖŠ¼ÓČė3mLŅŅ“¼£¬Č»ŗó±ßÕńµ“ŹŌ¹Ü±ßĀżĀż¼ÓČė
ijĶ¬Ń§ĪŖÖĘČ”ŅŅĖįŅŅõ„£¬ŌŚŹŌ¹ÜAÖŠ¼ÓČė3mLŅŅ“¼£¬Č»ŗó±ßÕńµ“ŹŌ¹Ü±ßĀżĀż¼ÓČė²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŹµŃéŹŅ³£ÓĆÅØĮņĖįŗĶŅŅ“¼»ģŗĻ¼ÓČČÖĘČ”ŅŅĻ©”£
£Ø1£©ŹµŃéŹŅÖĘŅŅĻ©µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
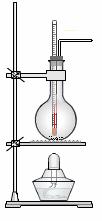 £Ø2£©ŹµŃéŹŅÓĆÅØĮņĖįŗĶŅŅ“¼»ģŗĻ¼ÓČČÖĘŅŅĻ©æÉÓĆČēÓŅĶ¼ĖłŹ¾×°ÖĆ,ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£
£Ø2£©ŹµŃéŹŅÓĆÅØĮņĖįŗĶŅŅ“¼»ģŗĻ¼ÓČČÖĘŅŅĻ©æÉÓĆČēÓŅĶ¼ĖłŹ¾×°ÖĆ,ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ ”£
A£®ÅØĮņĖįÖ»×÷“߻ƼĮ
B£®ŌŚ·“ӦȯĘ÷ÖŠ·ÅČė¼øʬĖé“Éʬ·ĄÖ¹»ģŗĻŅŗ±©·Š
C£®·“Ó¦ĪĀ¶Č»ŗĀżÉĻÉżÖĮ170”ę
D£®ÓĆÅÅĖ®·Ø»ņĻņĻĀÅÅĘų·ØŹÕ¼ÆŅŅĻ©
E£®Ō°µ×ÉÕĘæ֊װµĆŹĒ4mLŅŅ“¼ŗĶ12mL3mol/L H2SO4»ģŗĻŅŗ
F£®ĪĀ¶Č¼ĘÓ¦²åČė·“Ó¦ČÜŅŗŅŗĆęĻĀ£¬ŅŌ±ćæŲÖĘĪĀ¶Č
G£®·“Ó¦Ķź±ĻŗóĻČĻØĆš¾Ę¾«µĘ£¬ŌŁ“ÓĖ®ÖŠČ”³öµ¼¹Ü
£Ø3£©Čō½«“Ė×°ÖĆÖŠµÄĪĀ¶Č¼Ę»»³É·ÖŅŗĀ©¶·£¬Ōņ»¹æÉŅŌÖĘČ”µÄĘųĢåÓŠ£Ø¾Ę¾«µĘæÉÓĆæɲ»ÓĆ£© ”£
A£®CO2 B£®NH3
C£®O2 D£®SO2
E£®NO2 F£®Cl2
£Ø4£© ČēĪĀ¶Č¹żøߣ¬·“Ó¦ŗóČÜŅŗŃÕÉ«±ä ”£Ä³Ķ¬Ń§Éč¼ĘĻĀĮŠŹµŃéŅŌČ·¶ØÉĻŹö»ģŗĻĘųĢåÖŠŗ¬ÓŠŅŅĻ©ŗĶSO2”£
|
|
¢ŁI”¢II”¢III”¢IV×°ÖĆæÉŹ¢·ÅµÄŹŌ¼ĮŹĒ£ØĒė½«ĻĀĮŠÓŠ¹ŲŹŌ¼ĮµÄŠņŗÅĢīČėæÕøńÄŚ£©£ŗ
A”¢Ę·ŗģ B”¢NaOHČÜŅŗ C”¢ÅØĮņĖį D”¢ĖįŠŌKMnO4ČÜŅŗ
I £»II £»III £»IV ”£
¢ŚÄÜĖµĆ÷SO2ĘųĢå“ęŌŚµÄĻÖĻóŹĒ £»
¢ŪŹ¹ÓĆ×°ÖĆIIµÄÄæµÄŹĒ £»
¢ÜŹ¹ÓĆÖĆIIIµÄÄæµÄŹĒ £»
¢ŻČ·¶Øŗ¬ÓŠŅŅĻ©µÄĻÖĻóŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”«DŹĒ֊ѧ»ÆѧŹµŃéÖŠ³£¼ūµÄ¼øÖÖĪĀ¶Č¼Ę×°ÖĆŹ¾ŅāĶ¼”£
£Ø1£©Ēė“Ó¢Ł”«¢ßÖŠŃ”³ö±ŲŠėŹ¹ÓĆĪĀ¶Č¼ĘµÄŹµŃ飬°Ń±ąŗÅĢīČė×īŹŹŅĖµÄ×°ÖĆĶ¼A”«CµÄæÕøńÖŠ”£¢Ł¾ĘĒåŗĶÅØĮņĖį»ģŗĻ¼ÓČČÖĘŅŅĻ© ¢ŚµēŹÆøśĖ®·“Ó¦ÖĘŅŅČ² ¢Ū·ÖĄė±½ŗĶĻõ»ł±½µÄ»ģŗĻĪļ ¢Ü±½ŗĶäåµÄČ”“ś·“Ó¦¢ŻŹ³ŃĪŗĶÅØĮņĖį»ģŗĻ¼ÓČČÖĘĀČ»ÆĒā ¢ŽÅØŃĪĖįŗĶ¶žŃõ»ÆĆĢ»ģŗĻ¼ÓČČÖĘĀČĘų¢ß²ā¶ØijĪĀ¶ČĻĀĻõĖį¼ŲŌŚĖ®ÖŠČܽā¶Č

£Ø2£©Ń”ÓĆ×°ÖĆD×ö±½µÄĻõ»ÆŹµŃ飬DÖŠ³¤²£Į§¹ÜµÄ×÷ÓĆŹĒ______________”£
£Ø3£©ĪŖĮĖ¼ģŃéäåŅŅĶéÖŠµÄäåŌŖĖŲ£¬ŅŌĻĀ²Ł×÷ŗĻĄķµÄĖ³ŠņŹĒ __””£ØĢīŠņŗÅ£©”£
a£®¼ÓAgNO3 ČÜŅŗ”” b£®¼ÓNaOH ČÜŅŗ”” c£®¼ÓČČ”” d£®¼ÓĻ”ĻõĖįÖĮČÜŅŗĻŌĖįŠŌ
 £Ø4£©ÓŅĶ¼ŹĒŹµŃéŹŅÖĘŅŅĖįŅŅõ„µÄ×°ÖĆĶ¼£¬»Ų“šĻĀĮŠĪŹĢā”£
£Ø4£©ÓŅĶ¼ŹĒŹµŃéŹŅÖĘŅŅĖįŅŅõ„µÄ×°ÖĆĶ¼£¬»Ų“šĻĀĮŠĪŹĢā”£
¢ŁŅŅĖįÖŠ18OŗĶŅŅ“¼ÖŠ16O·¢Éśõ„»Æ·“Ó¦£¬
Éś³ÉĖ®µÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ_________”£
¢Ś³¤²£Į§µ¼¹ÜµÄ×÷ÓĆ________”£
¢Ū±„ŗĶNa2CO3ČÜŅŗ×÷ÓĆ___________”£
![]()
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com