
��8�֣���֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl����ClO����ClO3�����ֺ���Ԫ�ص����ӣ�����ClO����ClO3���������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��
��1��t1ǰ������������________���ѧʽ����
��2��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ�� ��
��3����ʯ�����к���Ca��OH��2�����ʵ����� mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� ��
| A��NaCl��Cl2 | B��NaCl��NaClO | C��NaClO3��NaClO4 | D��NaCl��NaClO3 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ����ʦ���С��ٴ�һ�и���12��������ѧ�Ծ����������� ���ͣ������
��7�֣���֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ(�����ķ�Ӧ��Ϊ���ȷ�Ӧ)���������к���Cl ��ClO
��ClO ��
�� �����ֺ���Ԫ�ص����ӣ�����C1O
�����ֺ���Ԫ�ص����ӣ�����C1O ��
�� �������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
�������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
��1��t1ǰ������������ (�ѧʽ)��
(2)t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ��
(3)��ʯ�����к���Ca(OH)2�����ʵ����� mol
(4)NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� (����ĸ)��
| A��NaCl��Cl2 | B��NaCl��NaClO |
| C��NaClO3��NaClO4 | D��NaCl��NaClO3 |
 + OH
+ OH ��������
�������� 


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ���Ĵ�ʡ����������ٸġ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij�о���ѧϰС��Ի�ԭ������ˮ�����ķ�Ӧ������п�ѧ̽������֪Ca(OH)2�ķֽ��¶�Ϊ580�棬������ˮ������Ӧ���¶�Ϊ900�棺������ͼ��ʾʵ��װ�ã������˻�ԭ������ˮ�����ķ�Ӧʵ�飬ʵ���й۲쵽����Һ�в����˴��������ݡ�
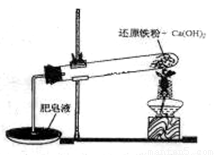
��1��ʵ����Ca(OH)2�������� ��ʵ���в�������������� ��
��2��Ϊ��һ��̽����ԭ������ˮ������Ӧ�������ijɷ֣��о���ѧϰС�齫��Ӧ��Ĺ��徭������õ���ɫ��������壬��Ժ�ɫ��������壬��С��������µļ��貢��������ص�ʵ�飺
����һ������ΪFeO
�����������ΪFe3O4
��������
����ѡ�������Լ������ᡢKSCN��Һ��K3Fe(CN)6 ��Һ����ˮ��֤������һ������
|
���� |
���� |
���� |
|
|
|
����һ������ |
��Ϊ�˽�һ��ȷ������ijɷ֣��ú�ɫ�������������ʵ��:

����������ˮ��������Ӧ�����ӷ���ʽ�� ��������ɫ�����Ƿ�ϴ�Ӹɾ��IJ����� ������ʱʢ�Ź���������� �����������������ɫ��������ȫ��ת���ɵĺ���ɫ��ĩ�������� g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ���ٴ�һ�и���12��������ѧ�Ծ��������棩 ���ͣ������
��7�֣���֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ(�����ķ�Ӧ��Ϊ���ȷ�Ӧ)���������к���Cl ��ClO
��ClO ��
�� �����ֺ���Ԫ�ص����ӣ�����C1O
�����ֺ���Ԫ�ص����ӣ�����C1O ��
�� �������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
�������ӵ����ʵ���(n)�뷴Ӧʱ��(t)����������ͼ��ʾ��
��1��t1ǰ������������ (�ѧʽ)��
(2)t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ��
(3)��ʯ�����к���Ca(OH)2�����ʵ����� mol
(4)NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� (����ĸ)��
A��NaCl��Cl2 B��NaCl��NaClO
C��NaClO3��NaClO4 D��NaCl��NaClO3
(5)��ƽ�������ӷ���ʽ�� Fe(OH)3+ ClO + OH
+ OH ��������
��������



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ������һ���¿���ѧ�Ծ��������棩 ���ͣ������
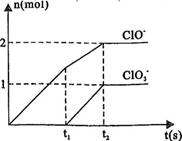
��8�֣���֪Ca(OH)2��Cl2��Ӧ�������������¶��йأ���һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ�������ķ�Ӧ��Ϊ���ȷ�Ӧ�����������к���Cl����ClO����ClO3�����ֺ���Ԫ�ص����ӣ�����ClO����ClO3���������ӵ����ʵ�����n���뷴Ӧʱ�䣨t����������ͼ��ʾ��

��1��t1ǰ������������________���ѧʽ����
��2��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵ����ӷ���ʽΪ�� ��
��3����ʯ�����к���Ca��OH��2�����ʵ����� mol��
��4��NaClO2���ȶ��������Ȼ��û��������ƹ���ʱ������ը���䱬ը��IJ�������� ��
A��NaCl��Cl2 B�� NaCl��NaClO C��NaClO3��NaClO4 D�� NaCl��NaClO3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com