
����Ŀ��������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ__��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ__��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����__��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ__���ṩ�µ��ӶԵijɼ�ԭ����__��
�۰��ķе�___������������������������좣�PH3����ԭ����__������__���ӣ����������������Ǽ�������������ԭ�ӵĹ���ӻ�����Ϊ__��
��3������ͭ����������__���γɵľ��壺Ԫ��ͭ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ/mol��INi=1753kJ/mol��ICu>INi��ԭ����__��
��4��ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
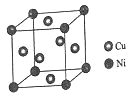
�پ�����ͭԭ������ԭ�ӵ�������Ϊ__��
�����Ͻ���ܶ�Ϊdg/cm3����������a=__nm��
���𰸡�1s22s22p63s23p63d84s2��3d84s2 2 �������� ��λ�� N ���� NH3���Ӽ���γ���� ���� sp3 ���� ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���� 3��1 ![]() ��107
��107
��������
��1������28��Ԫ�أ�λ�ڵ������ڢ��壬���ݺ�������Ų��������̬ԭ�ӵĵ����Ų�ʽΪ1s22s2 2p63s23p63d84s2��3d�ܼ���5���������ռ��5������������ͬ�ĵ��ӣ�ʣ��3�������ٷֱ�ռ������������������������෴������δ�ɶԵĵ�����Ϊ2���ʴ�Ϊ:1s22s22p63s23p63d84s2��2��
��2���ٸ��ݼ۲���ӶԻ�������,SO42�����������Ӷ�������4���µ��Ӷ���Ϊ(6+2-2��4)��2=0���������ӵ����幹�������������Ρ��ʴ�Ϊ:�������壻
�ڸ�����λ�����ص㣬��[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ��λ�����ṩ�µ��ӶԵijɼ�ԭ����N���ʴ�Ϊ:��λ����N ��
�۷��Ӽ������������Ӽ�������ǿ�������ķе����좣�PH3�������ݼ۲���ӶԻ������ۣ�������ԭ��N���������Ӷ�������3���µ��Ӷ���Ϊ(5-3)��2=1��������ԭ����sp3�ӻ������ӳ������Σ�����������IJ��ص������Ǽ��Է��ӡ�
�ʴ�Ϊ:���ڣ�NH3���Ӽ���γ���������ԣ�
��3��ͭ�������ڽ���������ͭ���������ɽ������γɵľ��壻ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ�����ICu>INi���ʴ�Ϊ:������ͭʧȥ����ȫ������3d10���ӣ���ʧȥ����4s1���ӣ�
��4���ٸ��ݾ�̯�����㣬������ͭԭ�Ӹ���Ϊ6��![]() =3����ԭ�ӵĸ���Ϊ8��
=3����ԭ�ӵĸ���Ϊ8��![]() =1����ͭ����ԭ�ӵ�������Ϊ3��1���ʴ�Ϊ:3��1��
=1����ͭ����ԭ�ӵ�������Ϊ3��1���ʴ�Ϊ:3��1��
�ڸ��������������þ��������ΪCu3Ni�����Ͻ���ܶ�Ϊdg��cm-3��������=m��V��������a=![]() ��107nm���ʴ�Ϊ:
��107nm���ʴ�Ϊ:![]() ��107��
��107��


 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ߴ���������Ϊ�ϳ���������Ԫ�������ϵ�ԭ�ϣ���ҵ�Ͽ�����Ȼ�������̷������̿���Fe��Al��Mg��Zn��Ni��Si��Ԫ�أ��Ʊ�����������ͼ��ʾ���ش��������⣺
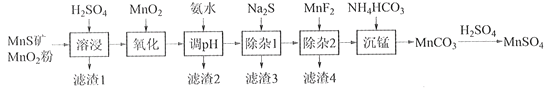
��ؽ�������[c0(Mn+)=0.1 mol��L1]�γ��������������pH��Χ���£�
�������� | Mn2+ | Fe2+ | Fe3+ | Al3+ | Mg2+ | Zn2+ | Ni2+ |
��ʼ������pH | 8.1 | 6.3 | 1.5 | 3.4 | 8.9 | 6.2 | 6.9 |
������ȫ��pH | 10.1 | 8.3 | 2.8 | 4.7 | 10.9 | 8.2 | 8.9 |
��1��������1������S��__________________________��д�����ܽ����ж������������̷�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
��2����������������������MnO2�������ǽ�________________________��
��3������pH��������������Һ��pH��ΧӦ����Ϊ_______~6֮�䡣
��4��������1����Ŀ���dz�ȥZn2+��Ni2+��������3������Ҫ�ɷ���______________��
��5��������2����Ŀ��������MgF2������ȥMg2+������Һ��ȹ��ߣ�Mg2+��������ȫ��ԭ����_____________________________________________________________________��
��6��д���������������ӷ���ʽ___________________________________________________��
��7����״��������Ԫ���Ͽ���Ϊ����ӵ���������ϣ��仯ѧʽΪLiNixCoyMnz2������Ni��Co��Mn�Ļ��ϼ۷ֱ�Ϊ+2��+3��+4����x=y=![]() ʱ��z=___________��
ʱ��z=___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������3.60gNaCl��NaHCO3��Na2CO3�Ļ�Ϲ��壬�����㹻��ʱ���������ʣ��3.29g����ʣ���������һ������������У�����0.448L����(��״����)������������Һϡ����100mL�����������ҺpH=1�������ж���ȷ���ǣ� ��
A.��Ϲ�����NaHCO3������Ϊ0.84g
B.��Ϲ�����Na2CO3������Ϊ2.12g
C.���������У�HCl�����ʵ���Ϊ0.04mol
D.����������Һ��c(Cl-)=0.1mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������ȡ����������Ҫ�������£�
�ٽ����ƺõ�Ũ������Ũ����Ļ���ᣬ��ȴ������Թ��С�
������������μ���һ�����ı����������Ͼ��ȡ�
��ˮԡ���ȷ�����Ӧ��
�ܷ�Ӧ���������Һ��ȴ��ת������Һ©���У���ȥ����ᣬ�ֲ�Ʒ����������ˮ��10%��Na2CO3��Һϴ�ӣ���������ˮϴ�ӣ��ô���������
�ݽ�������������ˮCaCl2���������õ�����������

�ش��������⣺
(1)����һ������Ũ�����Ũ����Ļ����IJ�����___��
(2)������У�ˮԡ���ȵ��ŵ���___��
(3)ͼ�г��������ܵ�������___��
(4)д����ȡ�������Ļ�ѧ����ʽ��___��
(5)������дֲ�Ʒ��10%��Na2CO3��Һϴ�ӵ�Ŀ����___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵ���������;���ж�Ӧ��ϵ����
A.ϡ���������ԣ������ڳ�ȥ����
B.Al2O3�������ԣ������ڵ��ұ����
C.SO2��ʹ���Ը��������Һ��ɫ��������Ư����ɫ����
D.��ȩ��Һ��ʹ�����ʷ������ԣ������ڶ���걾�ķ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��֧�ź�����ͷ�����ĸֹ�����������ӵ������������������з���������ԭ����ͼ��ʾ�����и߹�����Ϊ���Ը��������������йر�������ȷ����

A. ͨ�뱣������ʹ�ֹ����港ʴ�����ӽ�����
B. ͨ������·���ӱ�ǿ�ƴӸ߹���������ֹ�
C. �߹���������������Ϊ����������Ϻʹ��ݵ���
D. ͨ��ı�������Ӧ�ø��ݻ��������仯���е���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������˵����ȷ����
A.Na2S2O3��H2SO4��Һ��ϲ���22.4 L����ʱת�Ƶ�����Ϊ2NA
B.��״���£�22.4 L������ͱ�ϩ�Ļ���������������õ��Ӷ���Ϊ9NA
C.�����£�1 L 0.5 mol/L CH3COONH4��Һ��pH��7������Һ��CH3COO����NH4������Ŀ��Ϊ0.5NA
D.50g��������Ϊ46%���Ҵ���Һ���������Ʒ�Ӧ���ų�H2�ķ�����ĿΪ0.25NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ����Ͻ�����������ʹ�õĽ������ϡ�
��1����ͼ�ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ___________��

��2��Cu2������NH3��H2O��Cl�����γ���λ��Ϊ4������
�٣�Cu(NH3)4��2���д��ڵĻ�ѧ��������_____________������ţ���
A����λ�� B�������� C�����Թ��ۼ� D���Ǽ��Թ��ۼ� E�����Ӽ�
�ڣ�Cu(NH3)4��2�����жԳƵĿռ乹�ͣ���Cu(NH3)4��2���е�����NH3������Cl��ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ�����Cu(NH3)4��2���Ŀռ乹��Ϊ___________��
��3���������ڹ���Ԫ��Fe��Ti����C��H��N��O�γɶ��ֻ����
��H��C��N��O����Ԫ�صĵ縺����С�����˳��Ϊ_______________________��
��������������ȷ����____________��������ĸ��
A����ΪHCHO��ˮ���Ӽ����γ����������CH2O������ˮ
B��HCHO��CO2�����е�����ԭ�Ӿ�����sp2�ӻ�
C��C6H6�������6��![]() ����1����
����1����![]() ����C2H2�ǷǼ��Է���
����C2H2�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
�����ᣨHOCN����һ����״���ӣ����������ᣨHNCO����Ϊͬ���칹�壬������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ����д������Ľṹʽ________________��
��4��Feԭ�ӻ�������Χ�н϶���������Ŀչ������һЩ���ӻ������γ�������Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ������_________ д��һ���� CN- ��Ϊ�ȵ�����ĵ��ʷ���ʽ_______________________��
��5��һ��Al��Fe�Ͻ�����徧������ͼ��ʾ����ݴ˻ش��������⣺
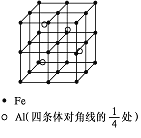
��ȷ���úϽ�Ļ�ѧʽ______________��
����������ܶȣ��� g/cm3����˺Ͻ������������Feԭ��֮��ľ���(�ú��ѵĴ���ʽ��ʾ�����ػ���)Ϊ__________cm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҩ��Ƴɷ��к���������ͪ�����ʡ��ӷ��͡��л��ӡ����ǵ�����ɷ֣�����һ���л��ӵĽṹ��ʽ��ͼ������˵����ȷ���� ( )
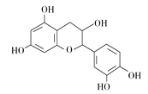
A. ����������̼ԭ�ӿ��Դ���ͬһƽ��
B. ����ʽΪC15H12O7
C. 1 mol���л��������Ũ��ˮ��Ӧ���������5 mol Br2
D. 1 mol���л�����NaOH��Һ��Ӧ�������5 mol NaOH
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com