
 ��֪����M��ͬһ�����ڵ�X��Y����Ԫ����ɣ�Xԭ�ӵ����������������ڲ��������һ�룬YԪ��������������ĸ��۴�����Ϊ6��M���������ʵ�ת����ϵ���£����ֲ�������ȥ����
��֪����M��ͬһ�����ڵ�X��Y����Ԫ����ɣ�Xԭ�ӵ����������������ڲ��������һ�룬YԪ��������������ĸ��۴�����Ϊ6��M���������ʵ�ת����ϵ���£����ֲ�������ȥ����
��1����ҵ���M��Һ�Ļ�ѧ����ʽΪ ��
��2����A��X��Yͬ���ڵ�һ�ֳ�����������AԪ�������ڱ��е�λ���� ���� �壬д��A��B��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��3����A��ijԪ�ص�һ�ֳ������������������������ά�����Ԫ��ԭ�ӽṹʾ��ͼΪ ��д��E��F��Ӧ�����ӷ���ʽ�� ��
��4��B�ĵ���ʽΪ��__________�����еĻ�ѧ��Ϊ��______________��
��5��˵��M���ʵ�һ����;��_________________��
��1��2NaCl + 2H2O 2NaOH + H2��+ Cl2��----------------------------2��
 ��2���������� IIIA�� 2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2��----------4��
��2���������� IIIA�� 2Al + 2NaOH + 2H2O = 2NaAlO2 + 3H2��----------4��
��3�� 2H+ + SiO32�� = H2SiO3��-----------------------3��
��4�� ���Ӽ��ͼ��Լ�---------------------4��
��5���ȼҵ���𰸺��������Ե÷֣�----------------------------1��


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
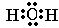
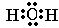
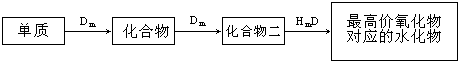
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
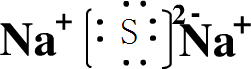
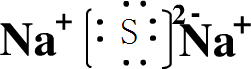
| ||
| ||
 HSO3-+OH-
HSO3-+OH- HSO3-+OH-
HSO3-+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��������ɳƺ�����Ͽ���ѧ�������ϣ��¿���ѧ�Ծ���12�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�궫����ʡ�����߿���ѧһģ�Ծ��������棩 ���ͣ������
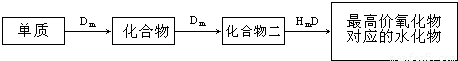
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com