
| O | - 2 |
| O | - 2 |
| O | 2- 6 |
| 90.5cV |
| 4m |
| 90.5cV |
| 4m |
| 250ml |
| 25ml |
| 250ml |
| 25ml |
| 90.5cV |
| 4m |
| 22.625cV |
| m |
| 90.5cV |
| 4m |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| 22.625cV |
| m |
| 22.625cV |
| m |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�����и�����ѧ��10���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ�����ȡNaClO2����
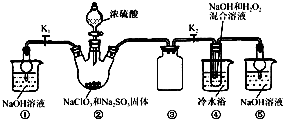
��֪��NaClO2������Һ���¶ȵ���38 ��ʱ�����ľ�����NaClO2��3H2O������38 ��ʱ�����ľ�����NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ10��ʾװ�ý���ʵ�顣

��1��װ�â۵�������____________________��
��װ�â��в���ClO2�Ļ�ѧ����ʽΪ____��
װ�â����Ʊ�NaClO2�Ļ�ѧ����ʽΪ____��
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55 �������ᾧ���ڳ��ȹ��ˣ���____________���ܵ���60 �����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-��4I����4H��=2H2O��2I2��Cl��)�������û��Һ���250 mL������Һ��
����ȡ25.00 mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol��L��1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL(��֪��I2��2S2O32-=2I����S4O62-)��
�ȴﵽ�ζ��յ�ʱ������Ϊ________________��
�ɸ���Ʒ��NaClO2����������Ϊ____________(�ú�m��c��V�Ĵ���ʽ��ʾ)��
���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������кӶ��������ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ��I����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ����Ʒ����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣

��1��װ�â۵�������
װ�âٵ�������
��2��װ�â��в���ClO2�Ļ�ԭ����
װ�â����Ʊ�ClO2�Ļ�ѧ����ʽΪ
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������ƷС���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-+4I-+4H+=2H2O+2I2+Cl-)�������û��Һ���250mL������Һ��
����ȡ25��00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol��L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL(��֪��I2+2S2O32-=2I-+S4O62-)��
��4���ﵽ�ζ��յ�ʱ������Ϊ
��5������Ʒ��NaClO2����������Ϊ (�ú�m��c��V�Ĵ���ʽ��ʾ)��
��6���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ̩���и�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧ��ȤС��ͬѧչ����Ư���������ƣ�NaClO2�����о���
ʵ�����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ����������NaClO2•3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣
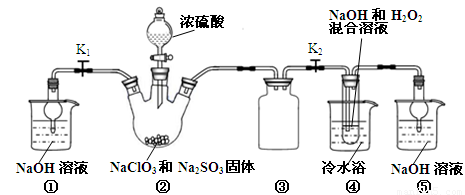
��1��װ�â۵������� ��
��2��װ�â��в���ClO2�Ļ�ѧ����ʽΪ ��װ�â����Ʊ�NaClO2�Ļ�ѧ����ʽΪ ��
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������Ʒm g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ����֪��ClO2��+ 4I��+4H+ =2H2O+2I2+Cl�����������û��Һ���250mL������Һ��
����ȡ25.00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol•L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL����֪��I2 +2S2O32��=2I��+S4O62������
��4���ﵽ�ζ��յ�ʱ������Ϊ ��
��5������Ʒ��NaClO2����������Ϊ ���ú�m��c��V�Ĵ���ʽ��ʾ����
��6���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com