
���� ��1�����ݴ����ƹ����ƽ��CH3COOH?CH3COO-+H+��Ӱ�������
��2����������ӽ��ˮ��������������ɴ��ᣬ��Ӧ�ǿ���ģ���������ӵ�ˮ�������ȷ�Ӧ��
��3����������Һ�����ʵ���Ũ��Խ��ˮ��̶�ԽС��������Һ������������Ũ��Խ����Һ��PHԽ��
��4����������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�
��5��pH��7��Һ��ʾ���ԣ���Һ�������Ӵ�������������Ũ�ȣ����������Ũ�ȴ���������Ũ�ȣ�
��6��pH=3��HA��Һ��������Ũ�Ⱥ�pH=11��NaOH��Һ�е�������������ȣ�������������ʣ�����������ǿ����ʣ�������Щ��ѡ������жϣ�
��7���������ӵ�Ũ�Ⱥ�����������Ũ�ȣ�������������ӵ����ӻ���Ȼ�����KSP[Mg��OH��2]=1.8��10-11��KSP[Zn��OH��2]=1.2��10-17��KSP[Cd��OH��2]=2.5��10-14�����ж��Ƿ����ɳ�����
��8������10mL 0.5mol•L-1������Һ������������ӵ����ʵ���Ũ�ȣ���Һϡ�ͣ����������ʵ������䣬�����ϡ�ͺ���Һ��������Ũ�ȣ�������Һ��ˮ����������ӵ�����Һ�����������ӵ�Ũ�ȣ�
��� �⣺��1��������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���C��H+����С��C��CH3COOH����������C��H+����C��CH3COOH���ı�ֵ��С���ʴ�Ϊ����С��
��2��������ˮ������ӷ���ʽΪ��CH3COO-+H2O?CH3COOH+OH-���÷�Ӧ�����ȷ�Ӧ���¶����ߣ���Һ������������Ũ������
�ʴ�Ϊ��CH3COO-+H2O?CH3COOH+OH-������
��3�����ڴ�������Һ�У������Ƶ�Ũ��Խ��ˮ��̶�ԽС��������Һ�е����������ӵ����ʵ���Ũ�ȷ���Խ����Һ��PHԽ������mС��n��a����b��
�ʴ�Ϊ��С�ڣ����ڣ�
��4������Ϊ������ʣ���������������ʵ���Ũ�ȵĴ��������������Һ��Ϻ�ǡ������CH3COONa��Ϊǿ�������Σ���Һ�ʼ��ԣ�c��OH-����c��H+������Һ�д��ڣ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+������c��Na+����c��CH3COO-����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
��5��������Һ��PHС��7����Һ�����������ʵ���Ũ�ȴ������������ӵ�Ũ�ȣ���������Ҫ�Ǵ������ģ����Դ��������Ũ�ȴ���������Ũ�ȣ�
�ʴ�Ϊ��С�ڣ�
��6��A������Ӧ����Һ�����ԣ���Һ��������Ũ��=����������Ũ��=1��10-7mol•L-1c��H+��+c��OH-��=2��10-7mol•L-1����A˵����ȷ��
B��������������ʲ��ֵ��룬�����Ũ�ȴ����������Ƶ�Ũ�ȣ���V1=V2����Ӧ����ҺpHһ��С��7����B˵������
C������Ӧ����Һ�����ԣ����ڴ���Ũ�ȴ�����������Ũ�ȣ���V1��V2����C˵������
D������Ӧ����Һ�ʼ��ԣ�����V1=V2��Һ��ʾ���ԣ�����V1һ��С��V2����D˵����ȷ��
��ѡ��BC��
��7����Һ������������Ũ���ǣ�[OH-]=2.2��10-5mol•L-1������[M2+][OH-]2=5��10-12��mol•L-1��3��
����5��10-12С��KSP[Mg��OH��2]=1.8��10-11��û��������þ�������ɣ�
����5��10-12����KSP[Zn��OH��2]=1.2��10-17����������п�������ɣ�
����5��10-12����KSP[Cd��OH��2]=2.5��10-14����Cd��OH��2�������ɣ�
�ʴ�Ϊ��Cd2+��Zn2+��C[OH-]=2.2��10-5mol•L-1��QC=c[M2+]��c[OH-]2=5��10-12��5��10-12����KSP[Zn��OH��2]=1.2��10-17��5��10-12����KSP[Cd��OH��2]=2.5��10-14��
��8��ϡ�ͺ���Һ�е�������Ũ���ǣ�c��H+��=$\frac{0.5mol•L{\;}^{-1}��0.01L}{0.5L}$=0.01mol/L������������Һ�У�ˮ�����������Ũ�ȵ�����Һ�е����������ӵ�Ũ�ȣ�����Һ����ˮ�������c��H+��=1��10-12��
�ʴ�Ϊ��1��10-12��
���� ���⿼��������ʵĵ���ƽ�⡢����ˮ�⡢����Ũ�ȱȽϡ���ҺPHֵ���жϵȣ��漰����Ŀ�����ϴ����Ѷ��еȣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ƹ�ʹ��̫���ܡ����ܵ������Դ���ܼ���PM2.5����Ⱦ | |
| B�� | ��ȼúͨ��������Һ���������仯���Լ�����Ⱦ�����ȼ��Ч�� | |
| C�� | ���ͷ��к��н϶��NaHCO3����ʹ���Ƴ��ĸ�����ɶ�� | |
| D�� | �����ơ�þ�Ȼ��ý����Ż�ʱ������ʹ����ĭ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ | B�� | ������ | C�� | ���� | D�� | ����������ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol | B�� | 0.15mol | C�� | 0.3mol | D�� | 0.5mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
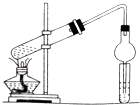 ���
���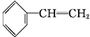 +Br2��
+Br2��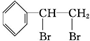 ��
��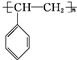 ��A��������ȫ�ӳɺ��һ�ȴ��ﹲ��6�֣�
��A��������ȫ�ӳɺ��һ�ȴ��ﹲ��6�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
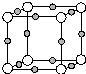 X��Y��Z��Q��W��N�˵�����������������Ԫ���У�Yԭ�Ӻ����L���������K���������Qԭ�Ӻ����L����ֻ������δ�ɶԵ��ӣ�X��Y��Q������ԭ�Ӹ���2��1��1��1�γɻ����W��Qͬ���壬Ԫ��N�ĵ����ܲ㹲��5�ԳɶԵ��ӣ���ش��������⣺
X��Y��Z��Q��W��N�˵�����������������Ԫ���У�Yԭ�Ӻ����L���������K���������Qԭ�Ӻ����L����ֻ������δ�ɶԵ��ӣ�X��Y��Q������ԭ�Ӹ���2��1��1��1�γɻ����W��Qͬ���壬Ԫ��N�ĵ����ܲ㹲��5�ԳɶԵ��ӣ���ش��������⣺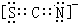 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
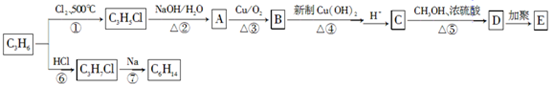
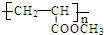 ��
�� +6NaBr��
+6NaBr���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com