
���������ΰ�һ��������Ϻ��ۣ����Ƶû�����X��X����ˮ�ܵ����K����Cr3+��SO42��������2.83 g X�е�Cr3+ȫ������ΪCr2O72������Һ�е�Cr2O72���ɺ���KI��Һ��Ӧ���õ�3.81g I2����Ӧ�����ӷ���ʽΪ��Cr2O72����6I����14H+��2 Cr3+��3I2��7H2O ����������2.83 gX����Һ�У����������BaCl2��Һ���ɵõ�4.66 g��ɫ�������ɴ˿��ƶϳ�X�Ļ�ѧʽΪ
A����K 2SO4 ��2Cr2��SO4��3�������� B�� 2K 2SO4 ��Cr2��SO4��3
C�� K 2SO4 ��Cr2��SO4��3�������� �� D�� K 2SO4 ��1/2Cr2��SO4��3
C
��������
�������������Cr3+Ԫ���غ�ɵù�ϵʽ��2Cr3+��Cr2O72����3I2��n(I2)= 3.81g��254g/mol=0.015mol������n(Cr3+)=0.01mol��������2.83 gX����Һ�У����������BaCl2��Һ���ɵõ�4.66 g��ɫ�����������S�غ�ɵ�n(SO42-)=n(BaSO4)=4.66 g��233g/mol=0.02mol;��������Һ�е���غ�1��n(K+)+ 3��n(Cr3+) = 2��n(SO42-)������n(K+)=2��0.02mol��3��0.01mol=0.01mol��n(K+)��n(Cr3+)��n(SO42-)=1:1:2�����Ի�ѧʽ��K 2SO4 ��Cr2��SO4��3��ѡ����C��
���㣺�����غ�ķ�����������ԭ��Ӧ�����ʵĻ�ѧʽ��ȷ���е�Ӧ�õ�֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ��һѧ�ڸ�����һ���¿���ѧ��A�����Ծ��������棩 ���ͣ�ѡ����
��ͼ��CO2�����ԭΪCH4�Ĺ���ԭ��ʾ��ͼ������˵������ȷ����
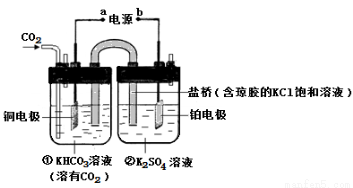
A���ù����ǵ���ת��Ϊ��ѧ�ܵĹ���
B��һ��ʱ��ٳ���n(KHCO3)����
C��һ��ʱ��ڳ�����Һ��pHһ���½�
D��ͭ�缫�ĵ缫��ӦʽΪCO2��8H+��8e-��CH4��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�ϲ��и���8���¿���ѧ�Ծ��������棩 ���ͣ������
(12��) ��1�������������ɷ�����H1N1���С�����������Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч�����Ĺ�����������������KClO3��H2SO4��������Na2SO3��Ӧ�Ƶá���д����Ӧ�����ӷ���ʽ___________________________________________��
��2��ij��ɫ��Һֻ��������8�������е�ij���֣�Na����H����Mg2����Ag����Cl����OH����HCO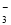 ��NO
��NO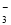 ����֪����Һ����Al2O3��Ӧ����
����֪����Һ����Al2O3��Ӧ����
�ٸ���Һ��Al2O3��Ӧ����Al3�����ɣ���ԭ��Һ��һ������____��һ�����Ậ�д�����________��
�ڸ���Һ��Al2O3��Ӧ����AlO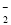 ���ɣ���ԭ��Һ��һ������________�����ܺ��д�����________��
���ɣ���ԭ��Һ��һ������________�����ܺ��д�����________��
��д������Һ��Al2O3��Ӧ����AlO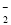 �����ӷ���ʽ__________________________________��
�����ӷ���ʽ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�ϲ��и���8���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����е����ⳣ�漰����ѧ֪ʶ��������������ȷ����
A�����ᱵ��һ��������ˮ������Σ�������X���ӳ�θ��ҩ��
B��ʹ���������Զ�ˮ����������ɱ��
C������Ʒ�ڸ���Ŀ�����������
D��������˿������˿����Ҫ�ɷ�����ά��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�ϲ�����У�����ϵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���±���ʾ��Ϊ�ᴿ�������ʣ�������Ϊ�������ʣ�����ѡ�õij����Լ�����Ҫ���뷽������ȷ����
| �������� | �����Լ� | ���뷽�� |
A | �����ױ��� | KMnO4���ữ����NaOH��Һ | ��Һ |
B | NH4Cl��Һ��FeCl3�� | NaOH��Һ | ���� |
C | �������������ᣩ | KOH��Һ��ˮ | ��Һ |
D | CO3��SO2�� | Na2CO3 | ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�ϲ�����У�����ϵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
��CO2��H2��CO��ɵĻ��������ͬ��ͬѹ���뵪�����ܶ���ͬ����û��������CO2��H2��CO�������Ϊ
A��29 ��8 ��13 B��22 ��1 ��14 C��13 ��8 ��29 D��26 ��15 ��57
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ������
(12��)������������������(��Ҫ�ɷ�ΪFe2O3��Fe3O4��FeO��SiO2)Ϊԭ���Ʊ��ߴ�����������������ʾ��ͼ��
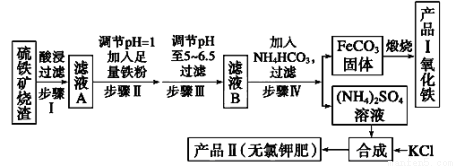

�ش��������⣺
��1����������˺���ҺA�еĽ�����������_______________________ _______��
��2����ҺB�м���NH4HCO3��Һ�����ӷ���ʽ ��
��3������FeCO3���ɲ�ƷI�Ļ�ѧ��Ӧ����ʽΪ___________ __________________��
��4����֪�����ε��ܽ�����¶ȱ仯����������ͼ��ʾ����Ʒ��Ļ�ѧʽΪ��������������Ϊ�˻�ò�Ʒ����(NH4)2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǡ������������������ȹ��ˡ�ϴ�ӡ����
��5�������Ʒ�������Ƿ����������Ȼ����������õ����Լ���_______________����һ���ᴿ��Ʒ��ķ�����________________��
��6��������п�ѡ��______________(�����)�Լ�������Һ��pH��
A��ϡ���� B��˫��ˮ C����ˮ D�����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ������(������������)�������Ӧʵ���һ����
ѡ�� | ʵ������(ʡ�Լг�װ��) | ��Ӧʵ�� l |
A | ���żܡ������ǡ�����������ǯ | ��CuSO4 |
B | �ձ�������������ͷ�ιܡ�����ƿ | ��Ũ��������0.1mol��L-1��HCl��Һ |
C | �ձ�������������Һ©�� | �ñ���Na2CO3��Һ��ȥ���������е�������Ҵ� |
D | �ձ�����ʽ�ζ��ܡ���ʽ�ζ��� | ��H2SO4��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭����У������һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��Na2CO3��Һ�м�������Ca(OH)2 ���壬CO32��ˮ��̶ȼ�С����Һ��pH ��С
B��25��ʱ���ô�����Һ�ζ���Ũ��NaOH��Һ��pH��7��V(����)��V(NaOH)
C����NaAlO2��Һ�еμ�NaHCO3��Һ���г�������������
D��CH3COOH ��Һ��ˮϡ�ͺ���Һ��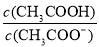 ��ֵ��С
��ֵ��С
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com