
ŌŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ£¬ČōĶØÖ±Į÷µē5 minŹ±£¬Ķµē¼«ÖŹĮæŌö¼Ó2.16 g”£ŹŌ»Ų“šĻĀĮŠĪŹĢā”£
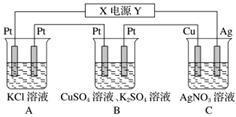
(1)µēŌ“ÖŠXµē¼«ĪŖÖ±Į÷µēŌ“µÄ________¼«”£
(2)pH±ä»Æ£ŗA£ŗ________£¬B£ŗ________£¬C£ŗ________(Ģī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±)”£
(3)Ķصē5 minŹ±£¬BÖŠ¹²ŹÕ¼Æ224 mL(±ź×¼×“æöĻĀ)ĘųĢ壬ČÜŅŗĢå»żĪŖ200 mL£¬ŌņĶصēĒ°CuSO4ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ________(Éčµē½āĒ°ŗóČÜŅŗĢå»żĪŽ±ä»Æ)”£
(4)ČōAÖŠKCl×ćĮæĒŅČÜŅŗµÄĢå»żŅ²ŹĒ200 mL£¬µē½āŗó£¬ČÜŅŗµÄpHĪŖ__________(Éčµē½āĒ°ŗóČÜŅŗĢå»żĪŽ±ä»Æ)”£
“š°ø””(1)øŗ””(2)Ōö“ó””¼õŠ”””²»±ä””(3)0.025 mol·L£1””(4)13
½āĪö””(1)Čżøö×°ÖĆŹĒ“®ĮŖµÄµē½ā³Ų”£µē½āAgNO3ČÜŅŗŹ±£¬Ag£«ŌŚŅõ¼«·¢Éś»¹Ō·“Ó¦±äĪŖAg£¬ĖłŅŌÖŹĮæŌö¼ÓµÄĶµē¼«ŹĒŅõ¼«£¬ŌņŅųµē¼«ŹĒŃō¼«£¬YŹĒÕż¼«£¬XŹĒøŗ¼«”£
(2)µē½āKClČÜŅŗÉś³ÉKOH£¬ČÜŅŗpHŌö“ó£»µē½āCuSO4ČÜŅŗÉś³ÉH2SO4£¬ČÜŅŗpH¼õŠ”£»µē½āAgNO3ČÜŅŗ£¬ŅųĪŖŃō¼«£¬²»¶ĻČܽā£¬Ag£«ÅØ¶Č»ł±¾²»±ä£¬pH²»±ä”£
(3)Ķصē5 minŹ±£¬CÖŠĪö³ö0.02 mol Ag£¬µēĀ·ÖŠĶعż0.02 molµē×Ó”£BÖŠ¹²ŹÕ¼Æ0.01 molĘųĢ壬ČōøĆĘųĢåČ«ĪŖŃõĘų£¬ŌņµēĀ·ÖŠŠčĶعż0.04 molµē×Ó£¬µē×Ó×ŖŅĘ²»ŹŲŗć”£Ņņ“Ė£¬BÖŠµē½ā·ÖĪŖĮ½øö½×¶Ī£¬Ļȵē½āCuSO4ČÜŅŗ£¬Éś³ÉO2£¬ŗóµē½āĖ®£¬Éś³ÉO2ŗĶH2£¬BÖŠŹÕ¼Æµ½µÄĘųĢåŹĒO2ŗĶH2µÄ»ģŗĻĪļ”£Éčµē½āCuSO4ČÜŅŗŹ±Éś³ÉO2µÄĪļÖŹµÄĮæĪŖx£¬µē½āH2OŹ±Éś³ÉO2µÄĪļÖŹµÄĮæĪŖy£¬Ōņ4x£«4y£½0.02 mol(µē×Ó×ŖŅĘŹŲŗć)£¬x£«3y£½0.01 mol(ĘųĢåĢå»żÖ®ŗĶ)£¬½āµĆx£½y£½0.002 5 mol£¬ĖłŅŌn(CuSO4)£½2”Į0.002 5 mol£½0.005 mol£¬c(CuSO4)£½0.005 mol”Ā0.2 L£½0.025 mol·L£1”£(4)Ķصē5 minŹ±£¬AÖŠ·Å³ö0.01 mol H2£¬ČÜŅŗÖŠÉś³É0.02 mol KOH£¬c(OH£)£½0.02 mol”Ā0.2 L£½0.1 mol·L£1£¬pH£½13”£


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
a mol CuÓėŗ¬b mol HNO3µÄČÜŅŗĒ”ŗĆĶźČ«·“Ó¦£¬±»»¹ŌµÄHNO3µÄĪļÖŹµÄĮæŅ»¶ØŹĒ(””””)
A£®(b£2a) mol B.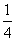 b mol
b mol
C.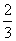 a mol D£®2a mol
a mol D£®2a mol
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
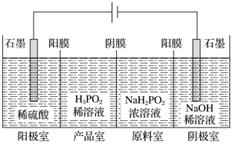
ÖŠ»ŖČĖĆń¹²ŗĶ¹ś¹ś¼Ņ±ź×¼(GB27602011)¹ę¶ØĘĻĢŃ¾ĘÖŠSO2×ī“óŹ¹ÓĆĮæĪŖ0.25 g·L£1”£Ä³ŠĖȤŠ”×éÓĆĢāĶ¼1×°ÖĆ(¼Š³Ö×°ÖĆĀŌ)ŹÕ¼ÆijĘĻĢŃ¾ĘÖŠSO2£¬²¢¶ŌĘäŗ¬Įæ½ųŠŠ²ā¶Ø”£
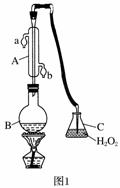

(1)ŅĒĘ÷AµÄĆū³ĘŹĒ______________£¬Ė®ĶØČėAµÄ½ųæŚĪŖ________”£
(2)BÖŠ¼ÓČė300.00 mLĘĻĢŃ¾ĘŗĶŹŹĮæŃĪĖį£¬¼ÓČČŹ¹SO2Č«²æŅŻ³ö²¢ÓėCÖŠH2O2ĶźČ«·“Ó¦£¬Ęä»Æѧ·½³ĢŹ½ĪŖ________________________________”£
(3)³żČ„CÖŠ¹żĮæµÄH2O2£¬Č»ŗóÓĆ0.090 0 mol·L£1NaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø£¬µĪ¶ØĒ°ÅÅĘųÅŻŹ±£¬Ó¦Ń”ŌńĢāĶ¼2ÖŠµÄ________£»ČōµĪ¶ØÖÕµćŹ±ČÜŅŗµÄpH£½8.8£¬ŌņŃ”ŌńµÄÖøŹ¾¼ĮĪŖ________£»ČōÓĆ50 mLµĪ¶Ø¹Ü½ųŠŠŹµŃ飬µ±µĪ¶Ø¹ÜÖŠµÄŅŗĆęŌŚæĢ¶Č”°10”±“¦£¬Ōņ¹ÜÄŚŅŗĢåµÄĢå»ż(ĢīŠņŗÅ)________(¢Ł£½10 mL£¬¢Ś£½40 mL£¬¢Ū<10 mL£¬¢Ü>40 mL)”£
(4)µĪ¶ØÖĮÖÕµćŹ±£¬ĻūŗÄNaOHČÜŅŗ25.00 mL£¬øĆĘĻĢŃ¾ĘÖŠSO2ŗ¬ĮæĪŖ________g·L£1”£
(5)øĆ²ā¶Ø½į¹ū±ČŹµ¼ŹÖµĘ«øߣ¬·ÖĪöŌŅņ²¢ĄūÓĆĻÖӊװÖĆĢį³öøĽų“ėŹ©________________________________________________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
øł¾Ż2CrO £«2H£«??Cr2O
£«2H£«??Cr2O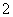 7£«H2OÉč¼ĘĶ¼Ź¾×°ÖĆ(¾łĪŖ¶čŠŌµē¼«)µē½āNa2CrO4ČÜŅŗÖĘČ”Na2Cr2O7£¬Ķ¼ÖŠÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄ______¼«£¬Ęäµē¼«·“Ó¦Ź½ĪŖ_________________________”£
7£«H2OÉč¼ĘĶ¼Ź¾×°ÖĆ(¾łĪŖ¶čŠŌµē¼«)µē½āNa2CrO4ČÜŅŗÖĘČ”Na2Cr2O7£¬Ķ¼ÖŠÓŅ²ąµē¼«Į¬½ÓµēŌ“µÄ______¼«£¬Ęäµē¼«·“Ó¦Ź½ĪŖ_________________________”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾µÄøÖĢśøÆŹ“ÖŠ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
 ””
””
A£®Ģ¼±ķĆę·¢ÉśŃõ»Æ·“Ó¦
B£®øÖĢś±»øÆŹ“µÄ×īÖÕ²śĪļĪŖFeO
C£®Éś»īÖŠøÖĢśÖĘĘ·µÄøÆŹ“ŅŌĶ¼¢ŚĖłŹ¾ĪŖÖ÷
D£®Ķ¼¢ŚÖŠ£¬Õż¼«·“Ó¦Ź½ĪŖO2£«4e££«2H2O===4OH£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
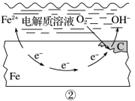
H3PO2Ņ²æÉÓƵēÉųĪö·ØÖʱø”£”°ĖÄŹŅµēÉųĪö·Ø”±¹¤×÷ŌĄķČēĶ¼ĖłŹ¾(ŃōĤŗĶŅõĤ·Ö±šÖ»ŌŹŠķŃōĄė×Ó”¢ŅõĄė×ÓĶعż)£ŗ
¢ŁŠ“³öŃō¼«µÄµē¼«·“Ó¦Ź½______________________________________________”£
¢Ś·ÖĪö²śĘ·ŹŅæɵƵ½H3PO2µÄŌŅņ__________________________________________
________________________________________________________________________ӣ
¢ŪŌēĘŚ²ÉÓĆ”°ČżŹŅµēÉųĪö·Ø”±ÖʱøH3PO2£ŗ½«”°ĖÄŹŅµēÉųĪö·Ø”±ÖŠŃō¼«ŹŅµÄĻ”ĮņĖįÓĆH3PO2Ļ”ČÜŅŗ“śĢę”£²¢³·Č„Ńō¼«ŹŅÓė²śĘ·ŹŅÖ®¼äµÄŃōĤ£¬“Ó¶ųŗĻ²¢ĮĖŃō¼«ŹŅÓė²śĘ·ŹŅ”£ĘäȱµćŹĒ²śĘ·ÖŠ»ģÓŠ____________ŌÓÖŹ”£øĆŌÓÖŹ²śÉśµÄŌŅņŹĒ________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĶÓėĻ”ĮņĖį²»·“Ó¦£¬Ä³Š£ŹµŃ銔×éµÄĶ¬Ń§ŌŚĄĻŹ¦µÄÖøµ¼ĻĀÉč¼ĘĮĖĻĀĮŠ×°ÖĆ£¬ŹµĻÖĮĖĶÓėĻ”ĮņĖįµÄ·“Ó¦”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
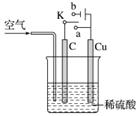
(1)¼×Ķ¬Ń§ČĻĪŖŌŚĶØČėæÕĘųµÄĶ¬Ź±£¬½«æŖ¹ŲKÓė______(Ģī”°a”±»ņ”°b”±)Į¬½Ó£¬¼“æÉŹµĻÖ”£Ōņ“ĖŹ±ŹÆÄ«µē¼«µÄ·“Ó¦Ź½ĪŖ__________________£¬µē³ŲµÄ×Ü·“Ó¦Ź½ĪŖ________________________”£µē³Ų¹¤×÷Ź±£¬H£«Ļņ________(Ģī”°C”±»ņ”°Cu”±)¼«ŅĘ¶Æ”£
(2)ŅŅĶ¬Ń§ČĻĪŖ£¬²»ĶØČėæÕĘų£¬½«KÓė______(Ģī”°a”±»ņ”°b”±)Į¬½Ó£¬Ņ²æÉŅŌŹµĻÖ”£ŌņCu¼«µÄµē¼«·“Ó¦Ź½ĪŖ________________________£¬×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________________”£Čō±ź×¼×“æöĻĀ²śÉś2.24 LĘųĢ壬ŌņµēĀ·ÖŠ×ŖŅʵĵē×ÓĪŖ______mol”£
(3)±ūĶ¬Ń§ČĻĪŖ»¹æÉŅŌÓĆČēĶ¼ĖłŹ¾×°ÖĆÄ£Äā¹¤ŅµÉĻµē¶ĘĶ”£ĖūČĻĪŖÖ»ŅŖ½«C»»³ÉFe(Cu×ćĮæ)£¬²¢½«ŅŅĶ¬Ń§µÄŹµŃé³ÖŠų×ć¹»³¤Ź±¼ä£¬¼“æÉŹµĻÖŌŚFeÉĻ¶ĘCu”£ÄćČĻĪŖĖūµÄĻė·Ø______(Ģī”°ÕżČ·”±»ņ”°²»ÕżČ·”±)£¬ĄķÓÉŹĒ______________”£ÕāÖÖ·½·ØµĆµ½µÄĶ¶Ę²ć______(Ģī”°ĄĪ¹Ģ”±»ņ”°²»ĄĪ¹Ģ”±)£¬ĄķÓÉŹĒ________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŅ“¼ŹĒÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬æÉÓÉŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·Ø»ņ¼ä½ÓĖ®ŗĻ·ØÉś²ś”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼ä½ÓĖ®ŗĻ·ØŹĒÖøĻČ½«ŅŅĻ©ÓėÅØĮņĖį·“Ӧɜ³ÉĮņĖįĒāŅŅõ„(C2H5OSO3H)£¬ŌŁĖ®½āÉś³ÉŅŅ“¼”£Š“³öĻąÓ¦·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________________________________________
________________________________________________________________________ӣ
(2)ŅŃÖŖ£ŗ
¼×“¼ĶŃĖ®·“Ó¦2CH3OH(g)===CH3OCH3(g)£«H2O(g)
¦¤H1£½£23.9 kJ·mol£1
¼×“¼ÖĘĻ©Ģž·“Ó¦2CH3OH(g)===C2H4(g)£«2H2O(g)
¦¤H2£½£29.1 kJ·mol£1
ŅŅ“¼Ņģ¹¹»Æ·“Ó¦C2H5OH(g)===CH3OCH3(g)
¦¤H3£½£«50.7 kJ·mol£1
ŌņŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·“Ó¦C2H4(g)£«H2O(g)===C2H5OH(g)µÄ¦¤H£½______________ kJ·mol£1”£
Óė¼ä½ÓĖ®ŗĻ·ØĻą±Č£¬ĘųĻąÖ±½ÓĖ®ŗĻ·ØµÄÓŵćŹĒ_____________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com