
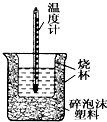
 50mL0.05mol•L-1������50mL0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL0.05mol•L-1������50mL0.55mol•L-1 NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5��������ʵ��������������к�����ָǿ���ǿ�Ӧ����1molˮʱ�ų��������������������أ�
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮����������ĭ���ϵ������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��4����Ӧ�ų����������������Լ�������Ķ����йأ���60mL 0.50mol•L-1�����50mL 0.55mol•L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5��һˮ�ϰ�Ϊ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС����õ��к�����ֵ��ƫС���к�����ָǿ���ǿ�Ӧ����1molˮʱ�ų��������������������أ���������50mL 0.50mol•L-1NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ��Ӱ�죻
�ʴ𰸣�ƫС����Ӱ�죮
���� ���⿼��ѧ���й��к��ȵIJⶨ֪ʶ��ע���к������ᡢ������ʵ����أ��ѶȲ���


 С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | x��O2�� | ��O2��Ӧ�� ��Q1/kJ�� | ��H2O��Ӧ�� ��Q2/kJ�� | �ܷ�Ӧ�� ��Q3/kJ�� |
| I | 0.2 | -18 | ||
| �� | -36 | +72 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۣ��٣��ܣ��� | B�� | �٣��ڣ��ۣ��� | C�� | �ۣ��ڣ��٣��� | D�� | �٣��ܣ��ۣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | CH3OH | B�� | C3H8 | C�� | C2H4 | D�� | CH4 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com