
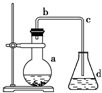
 ��ѧʵ��������ͼװ����ȡ�����屽������ƿa��װ���Լ��DZ���Һ������ۣ�d��װ��������ˮ����ش��������⣮
��ѧʵ��������ͼװ����ȡ�����屽������ƿa��װ���Լ��DZ���Һ������ۣ�d��װ��������ˮ����ش��������⣮���� ��1������Һ�����������������·���ȡ����Ӧ�����屽��
��2���������ӷ���
��3���屽����Һ�����ܣ����÷�Һ������ӣ�
��4�������巢������ȡ����Ӧ�����屽���廯�⣬�廯��������ˮ�����H+��Br-��ͨ������H+��Br-֤�������ڷ�Ӧ���ȣ�����Һ����ӷ�����Ĵ��ڸ�����H+��Br-��
��� �⣺��1������Һ�����������������·���ȡ����Ӧ�����屽����ѧ����ʽ��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr��
�ʴ�Ϊ��C6H6+Br2$\stackrel{Fe}{��}$C6H5Br+HBr��
��2���������ӷ�������b�������⣬�����������������ã����ٱ������ӷ���
�ʴ�Ϊ������������
��3��Ϊ�˳�ȥ���е��壬�������м�������NaOH��Һϴ�ӣ������������Ʒ�Ӧ���ɿ������廯����Һ���屽���廯����Һ�����ܣ����÷�Һ�����ᴿ��
�ʴ�Ϊ����Һ��
��4���������ȡ����Ӧ�������廯�⣬�廯��������ˮ�����H+��Br-��ֻҪ���麬�������ӻ������Ӽ��ɣ�
�����ӵļ��飺ȡ��Һ�μ���������Һ��������ɵ���ɫ������֤���������ӣ�
�����ӵļ��飺�����ʹ��ɫʯ����Һ��죬��֤�����������ӣ�
���ڷ�Ӧ���ȣ�����Һ����ӷ�����Ĵ��ڸ��ż���H+��Br-�����Ա����ڵ���a��b֮�����ϴ��ƿ����ȥ�廯�������е����������弫���������Ȼ�̼���л��ܼ���ϴ��ƿ��ʢ�����Ȼ�̼���ɣ�
�ʴ�Ϊ��AgNO3��Һ����ɫʯ����Һ�����Ȼ�̼����ȥ�廯�������е���������
���� ���⿼���˱������ʺ����ӵļ��飬��Ϥ����ȡ����Ӧԭ���������ӡ������Ӽ��鷽���ǽ���ؼ�����Ŀ�ѶȲ���


 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�2.24L�ױ��к���0.7NA��̼ԭ�� | |
| B�� | 7.8gNa2O2�����к��е���������Ϊ0.4NA | |
| C�� | 64gͭ������������۹��ȷ�Ӧ��ת�Ƶĵ�������Ϊ2NA | |
| D�� | ���³�ѹ�£�92g NO2��N2O4�Ļ�������к��е�ԭ������Ϊ6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��HPO42-����c��H3PO4�� | B�� | c��Na+��+c��H+��=0.1mol•L-1 | ||
| C�� | c��Na+��=c��H2PO4-��+c��HPO42-��+C��H3PO4�� | D�� | ��ˮϡ�ͺ�n��H+����n��OH-���ij˻����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������� SO42- ����Ũ�������� | |
| B�� | ������пƬ����ͭƬ | |
| C�� | ��Һ��pH��С | |
| D�� | ͭƬ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����½�0.1mol•L-1NH4Cl��Һ��0.05mol•L-1NaOH��Һ�������ϣ�c��Cl-����c��Na+����c��NH4+����c��OH-����c��H+�� | |
| B�� | ���������ʵ���Ũ����ȵĢ�NH4HSO4��CH3COONH4��NH4Cl������Һ��c��NH4+�����٣��ۣ��� | |
| C�� | 0.1mol•L-1��NaHA��Һ����pH=11������Һ�У�c��HA-����c��OH-����c��A2-����c��H2A�� | |
| D�� | ����ͬ�����£�������AgCl���������Ģ�0.01mol•L-1 KCl��0.1mol•L-1 KCl��Һ������ˮ����Һ���У������ܽ��AgCl������ϵΪ���٣��ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ�����û���Ӧ | |
| B�� | ${\;}_{\;}^{12}$C��${\;}_{\;}^{14}$C��̼Ԫ�ص�����ͬ�������� | |
| C�� | �÷�Ӧ�л�ѧ��ȫ��ת��Ϊ���� | |
| D�� | �ڸ÷�Ӧ�����£�C�Ļ�ԭ��ǿ��Mg�Ļ�ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com