
 ��������
��������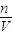 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ� =2mol�����ʵ���Ũ��C=
=2mol�����ʵ���Ũ��C=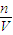 =
=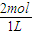 =2mol/L���ʴ�Ϊ��2mol/L��
=2mol/L���ʴ�Ϊ��2mol/L��

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£�1���ˮ�����ܽ�500�����HCl���塣����ˮ��ͨ���״���µ�44.8 L HCl�������1L��Һ������������ȫ�ܽ⣬����Һ�к�HCl�����ʵ���Ũ��Ϊ ��������Һ�ܶ�Ϊ1.1g/cm3������Һ�к�HCl��������Ϊ ���Ӹ���Һ��ȡ��10 mLŨ�����ܽ���ˮ���Ƴ�250 mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ��������� ��ѡ�����в�������ţ���
A������ƿ����ˮϴ��δ�Ӹ���
B����Ͳ������ˮϴ��δ����
C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶�
D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶���
E������ʱ������Һ���ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£�1���ˮ�����ܽ�500�����HCl���塣����ˮ��ͨ���״���µ�44.8 L HCl�������1L��Һ������������ȫ�ܽ⣬����Һ�к�HCl�����ʵ���Ũ��Ϊ ��������Һ�ܶ�Ϊ1.1g/cm3������Һ�к�HCl��������Ϊ ���Ӹ���Һ��ȡ��10 mLŨ�����ܽ���ˮ���Ƴ�250 mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ��������� ��ѡ�����в�������ţ���
A������ƿ����ˮϴ��δ�Ӹ���
B����Ͳ������ˮϴ��δ����
C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶�
D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶���
E������ʱ������Һ���ˮ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ���ڸ���ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��״���£�1���ˮ�����ܽ�500�����HCl���塣����ˮ��ͨ���״���µ�44.8LHCl�������1L��Һ������������ȫ�ܽ⣬����Һ�к�HCl�����ʵ���Ũ��Ϊ ��������Һ�ܶ�Ϊ1.1g/cm3������Һ�к�HCl��������Ϊ ���Ӹ���Һ��ȡ��10mLŨ�����ܽ���ˮ���Ƴ�250mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ��������� ��ѡ�����в�������ţ���
| A������ƿ����ˮϴ��δ�Ӹ��� |
| B����Ͳ������ˮϴ��δ���� |
| C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶� |
| D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��״���£�1���ˮ�����ܽ�500�����HCl���塣����ˮ��ͨ���״���µ�44.8 L HCl�������1L��Һ������������ȫ�ܽ⣬����Һ�к�HCl�����ʵ���Ũ��Ϊ ��������Һ�ܶ�Ϊ1.1g/cm3������Һ�к�HCl��������Ϊ ���Ӹ���Һ��ȡ��10 mLŨ�����ܽ���ˮ���Ƴ�250 mL��Һ�����ƺ��ϡ��Һ�к�HCl���ʵ���Ũ��Ϊ ������Ũ������������ϡ����ʱ�����������У�ʹ��ǰ�������Ƿ�©Һ�������� �����ƹ����У����Ũ��ƫ�͵IJ��������� ��ѡ�����в�������ţ���
A������ƿ����ˮϴ��δ�Ӹ���
B����Ͳ������ˮϴ��δ����
C�����ձ���Ũ������������ƿ��δ��ˮϴ���ձ������������м�ˮ���̶�
D���ý�ͷ�ι�������ƿ�м�ˮʱ�����������̶��ߣ������⽺ͷ�ιܴ�ƿ������������Һʹʣ����Һ���ɴ�̶���
E������ʱ������Һ���ˮ���̶���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com