
·ÖĪö £Ø1£©A£®¼×ĶéČ„µōŅ»øöĒāŌ×Ó²»ÄܵƵ½CH3+”¢CH3-£»
B£®Ō×Ó×ÜŹżĻąµČ”¢¼Ūµē×Ó×ÜŹż£Ø»ņµē×Ó×ÜŹż£©ĻąµČµÄĪ¢Į£»„ĪŖµČµē×ÓĢ壻-CH3£Ø¼×»ł£©”¢CH3-ÖŠCŌ×Ó¾łŠĪ³É3øö¦Ņ¼ü£¬¼×»łÖŠĢ¼Ō×ÓÓŠ1øöµ„µē×Ó£¬CH3-ÖŠCŌ×ÓÓɶŌ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæ¾łĪŖ4£»
C£®CH3-ÓėNH3”¢H3O+¾ł¾ßÓŠ4øöŌ×Ó”¢10øöµē×Ó£¬»„ĪŖµČµē×ÓĢ壬æÕ¼ä½į¹¹ĻąĖĘ£»
D£®Į½øö-CH3»ņŅ»øöCH3+ŗĶCH3-½įŗĻ¶¼ÄܵƵ½CH3CH3£»
£Ø2£©ZnŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d104s2£¬Ź§Č„4sÄܼ¶2øöµē×ÓŠĪ³ÉZn2+£»
ōČ»łÖŠCŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬ĘäĖüĢ¼Ō×ÓŠĪ³É4øö¦Ņ¼ü£¬¾łĆ»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ·Ö±šĪŖ3”¢4£»
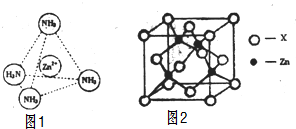
£Ø3£©Zn2+Ąė×ÓÓŠæÕ¹ģµĄ£¬NH3·Ö×ÓÓŠ¹Ā¶Ōµē×Ó£¬¶žÕßĶعżÅäĪ»¼üŠĪ³ÉÅäĄė×Ó[Zn£ØNH3£©4]2+£»
£Ø4£©øł¾Ż¾łĢƷؼĘĖć¾§°ūÖŠZnŌ×ÓŹżÄ攢XŌ×ÓŹżÄ棬½ų¶ųČ·¶Ø»ÆѧŹ½£®
½ā“š ½ā£ŗA£®¼×Ķé·Ö×Ó±ä³ÉCH3+”¢-CH3”¢CH3-Ź±£¬Ź§Č„µÄ·Ö±šŹĒĒāøŗĄė×Ó”¢ĒāŌ×ÓŗĶĒāĄė×Ó£¬¹ŹA“ķĪó£»
B£®CH3+”¢-CH3”¢CH3-·Ö±š¾ßÓŠ6øö”¢7øöŗĶ8øö¼Ūµē×Ó£¬²»ŹĒµČµē×ÓĢ壬-CH3£Ø¼×»ł£©”¢CH3-ÖŠCŌ×Ó¾łŠĪ³É3øö¦Ņ¼ü£¬¼×»łÖŠĢ¼Ō×ÓÓŠ1øöµ„µē×Ó£¬CH3-ÖŠCŌ×ÓÓɶŌ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæ¾łĪŖ4£¬¶žÕßĢ¼Ō×Ó²ÉČ”sp3Ōӻƣ¬¹ŹB“ķĪó£»
C£®CH3-ÓėNH3”¢H3O+¾ł¾ßÓŠ8øö¼Ūµē×Ó”¢4øöŌ×Ó£¬»„ĪŖµČµē×ÓĢ壬¼øŗĪ¹¹ŠĶ¾łĪŖČż½Ē׶ŠĪ£¬¹ŹCÕżČ·£»
D£®Į½øö-CH3»ņŅ»øöCH3+ŗĶCH3-½įŗĻ¶¼ÄܵƵ½CH3CH3£¬¹ŹDÕżČ·£»
¹ŹŃ”CD£»
£Ø2£©ZnŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ1s22s22p63s23p63d104s2£¬Ź§Č„4sÄܼ¶2øöµē×ÓŠĪ³ÉZn2+£¬Zn2+»łĢ¬µē×ÓÅŲ¼Ź½ĪŖ£ŗ1s22s22p63s23p63d10£¬
ōČ»łÖŠCŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬ĘäĖüĢ¼Ō×ÓŠĪ³É4øö¦Ņ¼ü£¬¾łĆ»ÓŠ¹Ā¶Ōµē×Ó£¬ŌӻƹģµĄŹżÄæĪŖ·Ö±šĪŖ3”¢4£¬·Ö×ÓÖŠĢ¼Ō×ÓŌӻƷ½Ź½ĪŖ£ŗsp2”¢sp3Ōӻƣ¬
¹Ź“š°øĪŖ£ŗ1s22s22p63s23p63d10£»sp2”¢sp3£»
£Ø3£©Zn2+Ąė×ÓÓŠæÕ¹ģµĄ£¬NH3·Ö×ÓÓŠ¹Ā¶Ōµē×Ó£¬¶žÕßĶعżÅäĪ»¼üŠĪ³ÉÅäĄė×Ó[Zn£ØNH3£©4]2+£¬ČēĶ¼ĖłŹ¾£ŗ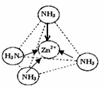 £¬
£¬
¹Ź“š°øĪŖ£ŗ £»
£»
£Ø4£©¾§°ūÖŠZnŌ×ÓŹżÄæĪŖ4”¢XŌ×ÓŹżÄæĪŖ8”Į$\frac{1}{8}$+6”Į$\frac{1}{2}$=4£¬ZnÓėXŌ×ÓŹżÄæÖ®±ČĪŖ1£ŗ1£¬¹Ź»ÆѧŹ½ĪŖZnX£¬
¹Ź“š°øĪŖ£ŗZnX£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬Éę¼°ŗĖĶāµē×ÓÅŲ¼Ź½”¢æռ乹ŠĶÓėŌӻƷ½Ź½µÄÅŠ¶Ļ”¢µČµē×ÓĢ唢ÅäŗĻĪļ”¢¾§°ū¼ĘĖćµČ£¬Ö¼ŌŚæ¼²éѧɜ¶Ō»ł“”ÖŖŹ¶µÄĄķ½āÓėÓ¦ÓĆ£¬ÄѶČÖŠµČ£®


 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 +Cl2
+Cl2
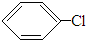 +HCl
+HCl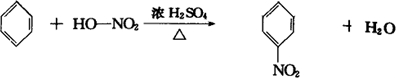
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½ŗĢåÄܲśÉś¶”“ļ¶ūŠ§Ó¦ | B£® | ½ŗĢå²»ÄÜĶعżĀĖÖ½ | ||
| C£® | ½ŗĢåĶā¹Ū²»¾łŌČ | D£® | ½ŗĢå²»ĪČ¶Ø£¬¾²ÖĆŗóČŻŅײśÉś³Įµķ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | äåŅŅĶéÖŠµĪČėAgNO3ČÜŅŗ¼ģŃéĘäÖŠµÄäåŌŖĖŲ£ŗBr-+Ag+ØTAgBr”ż | |
| B£® | ŹµŃéŹŅŅ»°ćÓĆ“æĖ®ŗĶµēŹÆÖĘŅŅČ²£ŗCaC2+2H2OØTC2H2”ü+Ca£ØOH£©2 | |
| C£® | ¼×±½ÓėÅØĻõĖįŗĶÅØĮņĖįµÄ»ģŗĻĪļ·“Ó¦£ŗ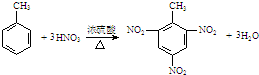 | |
| D£® | ŹµŃéŹŅÓĆŅŗäåŗĶ±½ÖĘäå±½£ŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | µē×ÓŌĘĶس£ŹĒÓĆŠ”ŗŚµćĄ“±ķŹ¾µē×ӵĶąÉŁ | |
| B£® | ŌŚĶ¬Ņ»Äܼ¶ÉĻŌĖ¶ÆµÄµē×Ó£¬ĘäŌĖ¶ÆדĢ¬æĻ¶ØĻąĶ¬ | |
| C£® | ÄܲćŠņŹżŌ½“ó£¬sµē×ÓŌʵİė¾¶Ō½“ó | |
| D£® | µē×Ó½öŌŚ¼¤·¢Ģ¬Ō¾Ēص½»łĢ¬Ź±²Å»į²śÉśŌ×Ó¹āĘ× |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
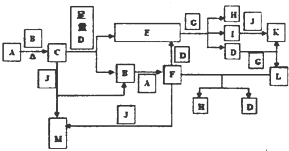
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCO3-+H2O?H3O++CO32- | B£® | PO43-+3H2O?H3PO4+3OH- | ||
| C£® | NH4++H2O?NH3H2O+H+ | D£® | H2O+H2O?H3O++OH- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | b=2a | B£® | V£ØŅŅĻ©£©=0.5aL | C£® | n£ØH2O£©=n£ØCO2£© | D£® | b=3a |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com