
����Ŀ��ijѧϰС������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������

��1��ʵ��ǰ���Ƚ�пͭ�Ͻ���ϡ�����н���Ƭ�̣���Ŀ���ǣ�___________________________________��
��2��ʵ����������У�����ҩƷ��ˮװ���������
�����Ӻ�װ�ú��������
����¼C��Һ��λ��
����B��ʣ�������ˣ�ϴ�ӣ��������
����B�в���������������ָ������º�
��¼C��Һ��λ��
����A��B�μ�������ϡ����
����ʵ�����ȷ����˳����________________������ţ�����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ_____________________
��3��B�з�����Ӧ�Ļ�ѧ����ʽΪ_________________ ��
��4����ʵ����пͭ�Ͻ������Ϊa g����ϡ�����ַ�Ӧ��
B��ʣ����������Ϊbg����п����������Ϊ____________��
��5��ʵ������У���δϴ�ӹ������õIJ��������п������������______������ƫ������ƫС����������Ӱ��������
���𰸡���ȥ�Ͻ���������Ĥ �ڢ٢ۢޢݢ� ʹD��C��Һ����ƽ Zn + H2SO4 = ZnSO4+H2�� (a��b)/a��100% ƫС
��������
(1)ʵ��ǰ,�Ƚ�пͭ�Ͻ���ϡ���н���Ƭ��,��ȥ�Ͻ���������Ĥ;
(2)
��ʵ����Ҫ���������������������ʵ�����Ӻ�װ�ú�������Ԣڣ�Ȼ��ҩƷ��ˮװ��������Т�����A��B�μ�������ϡ����Ҫ�����ſ�Һ���������ⶨ�������������,�������¼C��Һ��,Ȼ��ʹ��Ӧ����,����ַ�Ӧʱ�ڼ���C��λ�â�,Ȼ����A��B�μ�������ϡ���Ὺʼ��Ӧ�ޣ���Ӧ�����ⶨ��������ݣ����ⶨδ��Ӧ���������ܣ����Ա����Ϊ:�ڢ٢ۢޢݢ�;��¼C��Һ��λ��ʱ,������ƽ����,��Ӧ ʹD��C��Һ����ƽ��
(3)пͭ�Ͻ���п��ϡ���ᷴӦ����������ͭ����ϡ���ᷴӦ���ʷ�Ӧ����ʽΪ��Zn + H2SO4 = ZnSO4+H2����
(4)ͭп�Ͻ������Ϊag,B��ʣ����������Ϊbg,��п������Ϊ(a-b)g,�Ӷ��������п����������Ϊ(a-b)/a��100%��
(5)ʵ������У���δϴ�ӹ������õIJ������ⶨʣ�����ͭ������ƫ�����Բ��п������������ƫС��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƾ��壨Na2MoO4��2H2O��������������ȼ������������ˮϵͳ�Ľ������Ƽ�����ͼ�������⾫����Ҫ�ɷ���MoS2��������PbS�ȣ�Ϊԭ�����������ƾ���Ĺ�������ͼ��

�ش��������⣺
��1����߱���Ч�ʵķ�����____________����дһ�֣�
��2�������ա�ʱMoS2ת��ΪMoO3���÷�Ӧ���̵Ļ�ѧ����ʽΪ________________________������������________��д��ѧʽ����
��3���������ʱ���⻯���������Ҫ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
��4���������ؽ������ӡ�ʱ����ij�����ΪNa2S��������ɷֵĻ�ѧʽΪ________��
��5����á����ؽ������ӡ��в������ӵ�Ũ�ȣ�c(MoO42-)=0.40mol/L��c(SO42-)=0.04mol/L�����ᾧ��ǰ���ȳ�ȥSO42-�������Ǽ���Ba(OH)2���塣�������Ba(OH)2�������Һ������䣬��SO42-��ȫ������c(SO42-)��1.0��10-5mol/L��ʱ��BaMoO4�Ƿ��������____________________________________���������˵����[��֪��Ksp(BaSO4)=1.1��10-10��Ksp(BaMoO4)=4.0��10-8]
��6���⾫���ڼ��������£�����NaClO��Һ��Ҳ�����Ʊ������ƣ�ͬʱ��SO42-���ɣ��÷�Ӧ�����ӷ���ʽΪ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ŨH2SO4����480mL 0.2mol��L��1��ϡH2SO4��
��1����Ҫ��ȡ98%�ܶ�Ϊ1.84g��cm��3��Ũ����________mL��
��2������ʱ������ʹ�õ�������______(�����)����ȱ�ٵ�������_________��_________��
���ձ�����10mL��Ͳ����20mL��Ͳ����������ƽ�������룩���ݲ�����
��3������ʱ����ʵ�������õ��������������÷ֱ���________________��_______________��
��4�����ƹ����г��������������������ҺŨ���к�Ӱ�죨����ƫ��������ƫ����������Ӱ��������
������ƿû�и���________��
������Ͳ��ȡ98%��������Һʱ����________��
����Һת�Ƶ�����ƿ��δ����ϴ�Ӳ���_________ ��
�ܶ���ʱ��������ƿ__________��
�������ˮ�����˿̶��ߣ�ȡ��ˮʹҺ��ǡ�õ��̶���________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ԭ����1s22s22p63s23p2![]() 1s22s22p63s13p3ʱ��������ʶ��ȷ����
1s22s22p63s13p3ʱ��������ʶ��ȷ����
A. ��ԭ���ɻ�̬ת���ɼ���̬����һ��������������
B. ��ԭ���ɻ�̬ת���ɼ���̬����һ�������ͷ�����
C. ת����λ��p�ܼ��ϵ��������Ӵ���ͬһ����������������෴
D. ת�����ԭ������ԭ�ӵ��Ӳ�ṹ��ͬ����ѧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��X��Y��Z��T��W��ԭ��������������X�ĺ����������������������ͬ��Y��̬ԭ�ӵ�p��������s��������1����Z��̬ԭ�ӵļ۵��Ӳ�����2��δ�ɶԵ��ӣ�T��Zͬ���壬W��̬ԭ�ӵ�M��ȫ������N��ֻ��һ�����ӡ��ش��������⣺
��1��Y��Z��T�е����۵������______����Ԫ�ط��ţ�
��2����Wԭ��������5�Ļ�̬ԭ���е�������Ϊ_________________��
��3��T��Z�γɵĻ������У�����ԭ���ӻ�������_______
��4��X����������Ԫ���е�һ���γɵĻ�������:���ӳ������ε���________���ѧʽ���������мȺ��м��Թ��ۼ����ֺ��зǼ��Թ��ۼ��Ļ�������_______���ѧʽ��дһ�֣���
��5����5��Ԫ���γɵ�һ���������Ӹ�����Ϊ1:1�͵�������У������ӳ�������ṹ�������ӵĽṹ��ͼ��ʾ���������Ļ�ѧʽΪ__________���������д��ڵĻ�ѧ��������_______��
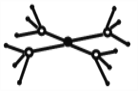
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������14.4gCO��CO2�Ļ������,�ڱ�״���������Ϊ8.96L.
��ش���������:
��1���û�������ƽ��Ħ������Ϊ_____.
��2�����������̼ԭ�ӵĸ���Ϊ_____(��NA��ʾ�����ӵ�������ֵ).
��3���������������ͨ����ͼ��ʾװ��,����ռ���������(����ڱ�״���²ⶨ).
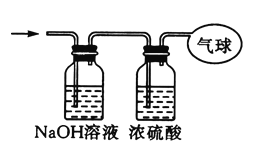
�������ռ����������Ħ������Ϊ_____.
�������ռ���������,��������Ϊ_____(��NA��ʾ�����ӵ�������ֵ).
����������Ϊ_____L.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�Ķ������������������ϣ�
����һ

���϶�
���� | �۵�/�� | �е�/�� | �ܶ�/ g/cm3 | �ܽ��� |
�Ҷ���(C2H6O2) | ��11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
������(C3H8O3) | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�ش���������(����ĸ���)��
A�������������������������� B����ȡ��
C�����ܽ⡢�ᾧ���������ķ��� D����Һ��
�ٽ�������Ȼ��ƺʹ���Ļ�����з�����������Ӧ��________��
�ڽ��Ҷ����ͱ�������������ѷ�����________________________��
(2)�Ķ�������
���ܽ��Է��棬Br2(��)��I2�����ƣ���ϡ��ˮ��Һ�Ի�ɫ����ʵ���������ˮ(Br2��ˮ��Һ)����ȡBr2����ȡI2�ķ������ơ�
�ش��������⣺
�ٳ��õ���ȡ������________________����ѧ�Լ���________������Ҫ��������__________��
�����۲췢����ȡBr2�Ժ��ˮ������ɫ�����������ķ�����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Na2CO3��10H2O��������240mL 0.1mol/L Na2CO3��Һ���ش��������⣺
��1����������ƽ��ȡNa2CO3��10H2O������Ϊ__________g(��ȷ��0.1g)
��2������������������������ƽ���ձ����������⣬����Ҫ_____��_______�����������ƣ���
��3������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�__________________��
A.������ˮϴ���ձ���������2-3�Σ�ϴ��Һ��ע������ƿ����
B.��������ƽ���������Na2CO3��10H2O���壬�����ձ��У��ټ�������ˮ���ò���������������ʹ����ȫ�ܽ�
C.������ȴ��Na2CO3��Һ�ز�����ע������ƿ��
D.������ƿ�ǽ�����ҡ��
E.���ý�ͷ�ιܼ�ˮ��ʹ��Һ��Һ�����͵�ǡ����̶�������
F.����������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1-2cm��
��4�������������������������Һ��Ũ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����Һע������ƿǰδ�ָ�������__________��
�ڳ���ʱ��Na2CO3��10H2O��ʧȥ���ֽᾧˮ_________��
��������ʱ���ӿ̶���_______��
�ܶ���ҡ��ʱ������Һ���½����ּ�ˮ���̶���_____________��
������ƿ������ˮϴ����û�и���_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б仯�У������ڻ�ѧ�仯���ǣ� ��
A.SO2ʹƷ����Һ��ɫB.��ˮʹ��ɫ������ɫ
C.����̿ʹ��īˮ��ɫD.Ư��ʹijЩȾ����ɫ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com