

 CH3��CH2��4COOCH2CH=CH2+H2O��
CH3��CH2��4COOCH2CH=CH2+H2O�� CH3��CH2��4COOCH2CH=CH2+H2O��
CH3��CH2��4COOCH2CH=CH2+H2O��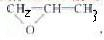 ����Ϊ��CH3OCH2CH2OH��������Ľṹ��ʽ��CH3OCH2CH��CH3��OOCCH2CH3���ʴ�Ϊ��CH3OCH2CH��CH3��OOCCH2CH3��
����Ϊ��CH3OCH2CH2OH��������Ľṹ��ʽ��CH3OCH2CH��CH3��OOCCH2CH3���ʴ�Ϊ��CH3OCH2CH��CH3��OOCCH2CH3��

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Ũ���� |
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������һģ ���ͣ������
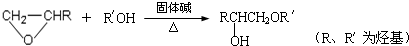

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
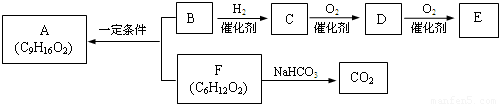
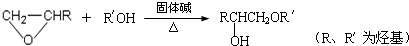
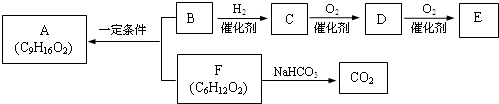
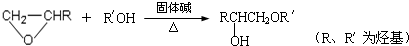
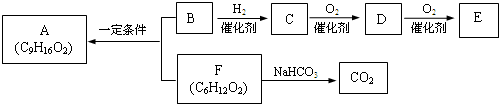
��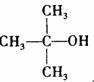 �ṹ���ƵĴ����ܱ�����Ϊȩ��ͪ���������͡��߷��������﹤������ �㷺��Ӧ��ǰ����PMAA����һ�֡������͡��߷��ӣ���Ӧ����������ҩ�д���Ӻ�С���ӵķ��롣������������AΪ��ʼ��Ӧ��ϳ�PMAA��·�ߣ�
�ṹ���ƵĴ����ܱ�����Ϊȩ��ͪ���������͡��߷��������﹤������ �㷺��Ӧ��ǰ����PMAA����һ�֡������͡��߷��ӣ���Ӧ����������ҩ�д���Ӻ�С���ӵķ��롣������������AΪ��ʼ��Ӧ��ϳ�PMAA��·�ߣ�
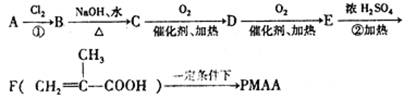
�ش��������⣺
��1���ֱ�д��A��PMAA�Ľṹ��ʽ��A ��PMAA ��
��2�����������Т١��ڵķ�Ӧ���ͷֱ��ǣ��� ���� ��
��3��д����ӦC��D�Ļ�ѧ����ʽ ��
��4��������G�������¹�ϵ��a. ��������֧��̼ԭ�ӣ�b. ��E������ͬ�Ĺ����ţ�����E��Ϊͬ���칹�壻c. �ɷ�����������ˮ������Ԫ���������H��д����ӦC��H�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com