
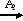 Na2CO3+CH4�� NH3��O2 ��2+1��
Na2CO3+CH4�� NH3��O2 ��2+1��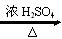 CH3COOCH2CH3+H2O ������Ӧ
CH3COOCH2CH3+H2O ������Ӧ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� |
| B����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| C������ŨH2SO4��ŨHNO3�Ļ���ʱ��Ӧ��H2SO4�����ӵ�ŨHNO3�У�����ʱ�������ȴ |
| D���þƾ���ȡ��ˮ�еĵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ױ�ʹ���Ը��������Һ��ɫ |
| B����֤̼������Աȱ���ǿ |
| C����ά�ص�ˮ�� |
| D���������Ҵ������������ռ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ����ҩƷʱ�����̷�ҩƷ�����̷����� |
| B�����Թ��еμ��Լ�ʱ�����ι��¶˽����Թ��ڱ� |
| C����ƿ���ձ��ھƾ�����ֱ�Ӽ��� |
| D��ʹ����ֽ������Һ������ʱ������ֽ������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ƽ����ʱ������ҩƷ������ֽ�ϣ�������������ƽ������ |
| B����Һʱ����Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ��� |
| C������С�̶�Ϊ0.1 mL����Ͳ��ȡ8.85 mL��Һ�� |
| D�����Թ����Һ�����ʱ���Թ����Ӧ����Թܿ�Ӧ������б |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������Ʊ�����ú���� |
| B���������Ʊ���������ƿ�� |
| C����״̼��ƹ��屣����ϸ�ڲ���ƿ�� |
| D��ϡ����ɱ�����ϸ��ĥɰ����ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������ԭCuOʱӦ��ͨ����������Ӧװ���еĿ����ž����ٵ�ȼ�ƾ��� |
| B������ʱ�¶ȼ�ˮ����Ӧ����������ƿ��֧�ܿ� |
| C�������ᾧʱ,���д�����������ʱҪ�ò��������Ͻ��貢������������Һ���� |
D������һ�����ʵ���Ũ�� ����Һ������,ҡ�Ⱥ���Һ����ڿ̶���,�����ٲ���ˮ ����Һ������,ҡ�Ⱥ���Һ����ڿ̶���,�����ٲ���ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ƭ���ڷ����ڲ��ܷ��ֽ���� |
| B������������Һʢ���ڴ�ĥ�ڲ�������ϸ��ƿ�� |
| C��FeSO4��Һ����ڼ����������۵��Լ�ƿ�� |
| D�������ƴ����ú���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com