
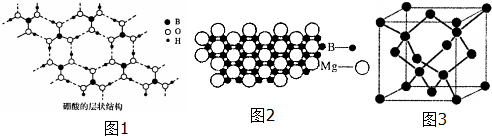
���� ��1��Ga��Bͬ���壬���ڵ������ڢ�A�壬���������Ϊ5+8+18=31����Ϻ�������Ų�������д��ͬ�������϶��µ�һ�����ܼ�С��
��2������ͼ��֪��Bԭ���γ�3��B-O�Ҽ���û�йµ��Ӷԣ��ݴ��ж�Bԭ���ӻ���ʽ��
�������ᾧ��ṹ��֪�����ڷ�����O��B��H֮���γɹ��ۼ������Ӽ�H��O֮���γ������
�ۼ����ƻ����������֮�����������������ˮ����֮���γ������
��������һԪ���ᣬ��ˮ�е���ʱ��������ˮ�������OH-�������ԣ�����������[B��OH��4]-��H+��
��3�����⻯�ƣ�NaBH4�����л���ѧ�е�һ�ֳ��û�ԭ��������ˮ��ˮ�����������ƺ��������ݴ���д����ʽ������[BH4]-��Bԭ�Ӽ۲���Ӷ�����µ��Ӷԣ�ȷ����ռ乹�ͣ�
��4��ԭ��������ͬ���۵���������ͬ������Ϊ�ȵ����壻
��5���þ����۽ṹ����ͼ�У�ÿ��Mgԭ����Χ��6��Bԭ�ӣ�ÿ��Bԭ��Ϊ3��Mgԭ�ӹ��ã����þ�̯��������
��6�����ʯ������Cԭ�Ӵ����������8�����㣬6�����ģ�������4�������ݾ�̯����֪�����к���8��Cԭ�ӣ�BN�Ļ�ѧʽ����֪�������ṹ����ʯ���ƣ�һ�������и�����4��Bԭ�ӡ�4��Nԭ�ӣ��������㵪���������������ܶ�=������������㵪������ܶȣ�
��� �⣺��1��Ga��Bͬ���壬���ڵ������ڢ�A�壬���������Ϊ5+8+18=31�����ݹ���ԭ��֪���̬ԭ�Ӻ�����ӷֲ�ʽΪ1s22s22p63s23p63d104s24p1��ͬ�������϶��µ�һ�����ܼ�С���ʵ�һ�����ܣ�B��Ga��
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1��B��Ga��
��2�������ȿ�֪��Bԭ���γ�3��B-O�Ҽ���û�йµ��Ӷԣ�Bԭ���ӻ������ĿΪ3��Bԭ�Ӳ�ȡsp2�ӻ���ʽ���ʴ�Ϊ��sp2��
�������ᾧ��ṹ��֪�����ڷ�����O��B��H֮���γɹ��ۼ������Ӽ�H��O֮���γ�����������֮��Ϊ���»������ʴ�Ϊ�����ۼ��������
�ۼ����ƻ����������֮�����������������ˮ����֮���γ����������ʱ��������ܽ������
�ʴ�Ϊ�������ƻ����������֮�����������������ˮ����֮���γ������
��������һԪ���ᣬ��ˮ�е���ʱ��������ˮ�������OH-�������ԣ�����������[B��OH��4]-��H+�����뷽��ʽΪ��H3BO3+H2O?[B��OH��4]-+H+��
�ʴ�Ϊ��H3BO3+H2O?[B��OH��4]-+H+��
��3�����⻯�ƣ�NaBH4�����л���ѧ�е�һ�ֳ��û�ԭ��������ˮ��ˮ�����������ƺ���������Ӧ����ʽΪ��NaBH4+4H2O�TNa[B��OH��4]+4H2����NaBH4+2H2O�TNaBO2+4H2����
[BH4]-��Bԭ�Ӽ۲���Ӷ���Ϊ4+$\frac{3+1-1��4}{2}$=4��û�йµ��Ӷԣ���ռ乹��Ϊ�������壬
�ʴ�Ϊ��NaBH4+4H2O�TNa[B��OH��4]+4H2����NaBH4+2H2O�TNaBO2+4H2�����������壻
��4��ԭ��������ͬ���۵���������ͬ������Ϊ�ȵ����壬1��B��Nԭ���൱��2��Cԭ�ӣ���B3N3H6��һ�ֵȵ��������ʵķ���ʽΪ��C6H6���ʴ�Ϊ��C6H6��
��5���þ����۽ṹ����ͼ�У�ÿ��Mgԭ����Χ��6��Bԭ�ӣ�ÿ��Bԭ��Ϊ3��Mgԭ�ӹ��ã���һ��Mgԭ�ӵ�Bԭ��Ϊ$\frac{1}{3}$��6=2��Mg����ԭ�Ӹ�����Ϊ1��2������þ�Ļ�ѧʽΪMgB2���ʴ�Ϊ��MgB2��
��6�����ʯ������Cԭ�Ӵ����������8�����㣬6�����ģ�������4�������ʯ������̼ԭ����ĿΪ��4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8��BN�Ļ�ѧʽ����֪�������ṹ����ʯ���ƣ�һ�������и�����4��Bԭ�ӡ�4��Nԭ�ӣ���������Ϊ4��$\frac{25}{{N}_{A}}$g������������ǣ�361.5��10-10��3cm3����������������ܶ�=4��$\frac{25}{{N}_{A}}$g�£�361.5��10-10��3cm3=$\frac{4��25}{��361.5��1{0}^{-10}��^{3}��{N}_{A}}$g•cm-3��
�ʴ�Ϊ��$\frac{4��25}{��361.5��1{0}^{-10}��^{3}��{N}_{A}}$��
���� �����Ƕ����ʽṹ�Ŀ��飬����ƴ������Ŀ���漰֪ʶ��϶࣬��Ҫѧ���߱���ʵ�Ļ�������ʵǨ�������������Ѷ��еȣ�


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��Fe3+��SO42-��SCN- | B�� | Na+��Mg2+��NO3-��OH- | ||
| C�� | K+��H+��Cl-��OH- | D�� | K+��H+��NO3-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������Ԫ��X��Y��Z��Wԭ��������������X�⻯���ˮ��Һ�Լ��ԣ�Y��Ԫ�����ڱ���������������������������ȣ�Z�����ǽ�̫����ת��Ϊ���ܵij��ò��ϣ�W����Ҫ�ġ�����Ԫ�ء�����Ҫ�����ε���ʽ�����ں�ˮ�У���ش�
������Ԫ��X��Y��Z��Wԭ��������������X�⻯���ˮ��Һ�Լ��ԣ�Y��Ԫ�����ڱ���������������������������ȣ�Z�����ǽ�̫����ת��Ϊ���ܵij��ò��ϣ�W����Ҫ�ġ�����Ԫ�ء�����Ҫ�����ε���ʽ�����ں�ˮ�У���ش�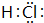 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 20mL 12mol/L������ | B�� | 10mL 18mol/L������ | ||
| C�� | 80 mL 2mol/L������ | D�� | 40 mL 14mol/L������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
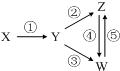 ���и��������У���һ��ʵ����ͼ��ʾ�١���ת����ϵ���ǣ�������
���и��������У���һ��ʵ����ͼ��ʾ�١���ת����ϵ���ǣ�������| X | Y | Z | W | |
| A | Fe3O4 | Fe | FeCl2 | FeCl3 |
| B | Al | Al2O3 | NaAlO2 | Al��OH��3 |
| C | H2SO4 | SO2 | S | SO3 |
| D | CH3CH2Br | CH2=CH2 | C2H5OH | CH2BrCH2Br |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HC��C-CH3 | B�� | 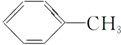 | C�� | 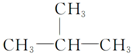 | D�� | 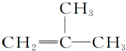 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ҵ���Ũ������·���������Ӧ | |
| B�� | ʹ������Ȼ�̼��Һ��ɫ | |
| C�� | ��������Һ��Ӧ������ | |
| D�� | ������������ͭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba��OH��2 | B�� | H2O2 | C�� | Na2O2 | D�� | CaCl2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com