
| A£®ĻõĖįļ§¹ĢĢåŗĶĒāŃõ»ÆøĘ¹ĢĢå»ģŗĻ¼ÓČČ | B£®¼ÓČČĀČ»Æļ§¹ĢĢå |
| C£®ĀČ»Æļ§¹ĢĢåŗĶĒāŃõ»ÆøĘ¹ĢĢå»ģŗĻ¼ÓČČ | D£®½«ÅØĮņĖįµĪČėÅØ°±Ė®ÖŠ |
 4NO + 6H20£»4NO + 3O2 + 2H2O = 4HNO3ÉčŌ»ģŗĻĘųĢåÖŠÓŠx mol O2£¬×īŗóČŻĘ÷ÖŠÉś³Éy mol HNO3£¬ŌŁĻņČŻĘ÷֊עČėŅ»¶ØĮæµÄH2O£¬µĆµ½ČÜŅŗ”£
4NO + 6H20£»4NO + 3O2 + 2H2O = 4HNO3ÉčŌ»ģŗĻĘųĢåÖŠÓŠx mol O2£¬×īŗóČŻĘ÷ÖŠÉś³Éy mol HNO3£¬ŌŁĻņČŻĘ÷֊עČėŅ»¶ØĮæµÄH2O£¬µĆµ½ČÜŅŗ”£
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
| ŹµŃé | Ņ©Ę· | ÖĘČ”ĘųĢå | ĮæĘų¹ÜÖŠµÄŅŗĢå |
| ¢Ł | Cu”¢Ļ”HNO3 | | H2O |
| ¢Ś | NaOH¹ĢĢ唢ÅØ°±Ė® | NH3 | |
| ¢Ū | Na2CO3¹ĢĢ唢Ļ”H2SO4 | CO2 | |
| ¢Ü | Ć¾ĀĮŗĻ½š”¢NaOHČÜŅŗ£Ø×ćĮ棩 | H2 | H2O |
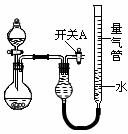
 3£©ŹµŃé¢ŚÖŠŹ£ÓąµÄNH3ŠčĪüŹÕ“¦Ąķ”£ŅŌĻĀø÷ÖÖĪ²ĘųĪüŹÕ×°ÖĆÖŠ£¬ŹŹŗĻÓŚĪüŹÕNH3£¬¶ųĒŅÄÜ·ĄÖ¹µ¹ĪüµÄÓŠ
3£©ŹµŃé¢ŚÖŠŹ£ÓąµÄNH3ŠčĪüŹÕ“¦Ąķ”£ŅŌĻĀø÷ÖÖĪ²ĘųĪüŹÕ×°ÖĆÖŠ£¬ŹŹŗĻÓŚĪüŹÕNH3£¬¶ųĒŅÄÜ·ĄÖ¹µ¹ĪüµÄÓŠ 
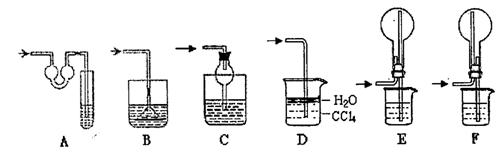
| A£®H2O | B£®CCl4 | C£®±„ŗĶNa2CO3ČÜŅŗ”””” | D£®±„ŗĶNaHCO3ČÜŅŗ |
| ±ąŗÅ | Ć¾ĀĮŗĻ½šÖŹĮæ | ĮæĘų¹ÜµŚŅ»“Ī¶ĮŹż | ĮæĘų¹ÜµŚ¶ž“Ī¶ĮŹż |
| ¢Ł[ | 1.0g | 10.0mL | 346.3mL |
| ¢Ś | 1.0g | 10.0mL | 335.0mL |
| ¢Ū | 1.0g | 10.0mL | 345.7mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
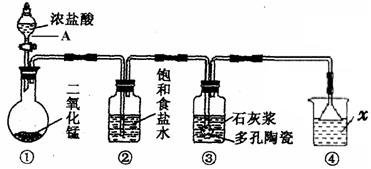
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
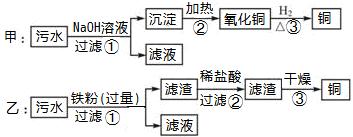
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
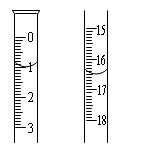
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com