
��ͼΪ��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ�����ֲ�������ȥ������֪��A��C��D�dz��������嵥�ʣ�F���弫������ˮ����Һ̬�����������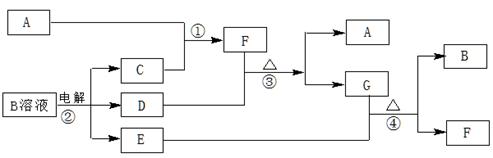
��1��д����ѧʽA ��D ��F ��G���� ���壻
��2������G�������ӵ�ʵ�鷽��������__________________________________��
��3��D���ʺ�E��Һ��Ӧ������һ�ֳ�������������Ư������Ч�ɷ֣�д��D+E��Һ��Ӧ�����ӷ���ʽ�͢۵Ļ�ѧ����ʽ �� ��
��4����ҵ�ϵ��B��Һ�Ƶ�һϵ�л���ԭ�ϣ�д������Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ ��
��1��N2��Cl2��NH3�����Ӿ��壻 ��2��ȡ����G����Һ�����Թ��У��ý�ͷ�ιܼ�������NaOHŨ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���Թܿڼ���ų������壬��ֽ����ɫ ��3��2OH��+Cl2=ClO��+Cl��+H2O
8NH3+3Cl2 ==N2+6NH4Cl
��4��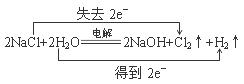
���������������1������F���弫������ˮ����Һ̬�����������֪FΪNH3�����ݷ�Ӧ��ͼ��֪AΪN2��CΪH2��DΪCl2��BΪNaCl��EΪNaOH��GΪNH4Cl���������Ӿ��塣
��2��G��������ΪNH4+������NH4+��NaOH��Ӧ����NH3��ԭ�����飬���Լ��鷽��������Ϊ����ȡ����G����Һ�����Թ��У��ý�ͷ�ιܼ�������NaOHŨ��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���Թܿڼ���ų������壬��ֽ����ɫ��
��3��Cl2��NaOH��Ӧ����NaCl��NaClO��H2O�����ӷ���ʽΪ��2OH��+Cl2=ClO��+Cl��+H2O����Ӧ��ΪCl2��NH3��Ӧ����N2��NH4Cl����ѧ����ʽΪ��8NH3+3Cl2 ==N2+6NH4Cl��
��4�����NaCl��Һ����Cl2��H2��NaOH����ƽ�ɵû�ѧ����ʽ�����ݻ��ϼ۵ı仯�ɱ������ת�Ƶķ������Ŀ��
���㣺���⿼��������ƶϡ��������͡����ӵļ��顢����ʽ����д������ת�ơ�


 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�е�ÿ1�����ʾһ���йصķ�Ӧ������������A��CΪ��ɫ���塣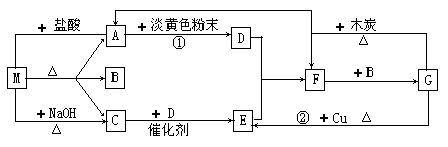
����������⣺
��1������M������ ��F�� ��
��2����Ӧ�ٳ�����D�⣬���ɵ���һ�����ʵ�ˮ��Һ�Լ��ԣ������ӷ���ʽ������Һ�Լ��Ե�ԭ�� ��
��3������M�������ӵķ����� ��
��4��G��E�����ӷ�Ӧ����ʽ�� ��ÿ���ɱ����1.12L��E��ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�� E��F�������ʵ��ת����ϵ����ͼ��ʾ����Ӧ����δ����������з�Ӧ�����û���Ӧ��B��C��F������̬���ʣ���BΪ����ɫ����Ӧ������ˮ���ɣ���Ӧ����Ҫ�ŵ���ܷ�����A�ǡ��ּ�������ˮ�����壬A��D�����а������ɡ�
(1)��Ӧ�۵Ļ�ѧ����ʽΪ_______________________________________________��
(2)��Ӧ����ÿ����1 molC��ת�Ƶĵ�����Ϊ_______________________________��
(3)A��D��ˮ��Һǡ����ȫ��Ӧʱ�����������ˮ��Һ����___________����ᡱ������С���������ˮ��Һ�д��������¹�ϵ��������Ũ�ȷ�����д��
��c(H��)+_________��c(OH��)+_____________��
��c(H��)��c(OH��)+_____________��
(4)Ԫ��X�����B��Ԫ��ͬ���ڣ�X�ĵ��ʼȿ����ᷴӦҲ����Ӧ�Ҷ�����H2����
��X�ĵ�����Ӧ�����ӷ���ʽ____________________________________��
��X����������ˮ����������ͺ�ˮ��־�Ƶĵ�ء����ֵ��Ժ�ˮΪ�������Һ���������е�����ʹX����������ԴԴ���ϲ���������������͵�ص������ĵ缫��ӦʽΪ___________________________��ԭ��ص��ܷ�Ӧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��M��N�����ֶ�����Ԫ�أ�ԭ��������������Xԭ����û�����ӣ�YԪ����������������֮��Ϊ0��M��Xͬ���壻Z��N�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
��ش��������⣺
��1������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳���ǣ�дԪ�ط��ţ�
��2��X��Y���γɶ��ֻ�������мȺ����Լ��ֺ��Ǽ��Լ�������Է���������С�����ʣ�д����ʽ�� ����M���ӵĵ����Ų�ʽΪ ��
������ijЩԪ����ɵĻ�����A��B��C��D������ת����ϵ
����C������ˮ�����Ե����壻D�ǵ���ɫ���塣
��3��д��C�Ľṹʽ ��D�ĵ���ʽ
��4�����A��B��������Ԫ����ɣ�BΪ���Բ����д����Aת��ΪB�����ӷ���ʽ
��5�����A������Ԫ����ɣ�B������Ԫ����ɣ�A��B��Һ���Լ��ԡ������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ�� ��
��6�������27����A��BŨ�Ⱦ�Ϊ0.1mol/L����Һ��ϣ������Һ������Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪C��D��G��IΪ������Ԫ���γɵĵ��ʣ�D��G��I������Ϊ��̬����GΪ����ɫ���γ�D��Ԫ��ԭ�ӵ������������Ǵ�����3����B����ɫ��Ӧ����ɫ������ɫ�ܲ�������KΪ����ɫ��ĩ����ת����ϵ��ͼ��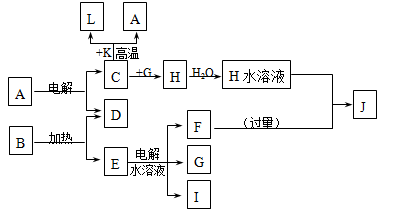
��ش�
��1����ҵ����C��A����H��ԭ��
��2��д��C��K��Ӧ�Ļ�ѧ����ʽ ���÷�Ӧ�ķ�Ӧ�������� ������ڡ���С�ڡ���������������
��3��L��ĿǰӦ����㷺�Ľ�������̼����������L����������ͨ��Դ����ʱ�䣩���Eˮ��Һ�Ļ�ѧ����ʽ ��
��4��J��H��Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����и��仯�У���Ӧ��Ϊ�����µķ�Ӧ��A��C��D������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬E������Ϊ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ���塣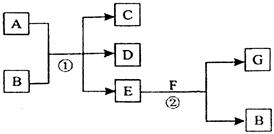
�ش��������⣺
��1��A��G�Ļ�ѧʽ�ֱ�Ϊ___ ��___ ��
��2��A��E��Ӧ�Ļ�ѧ����ʽΪ__ ��
��3��д����Ӧ�ٵĻ�ѧ����ʽ_____________��
��4���ڷ�Ӧ���У�ÿ����2.24 L����G(��״��)ʱ������F _ ___g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��G����Ԫ�ؾ��Ƕ�����Ԫ�أ���ԭ��������������Aԭ�������ӣ� B��Gԭ�ӵ�������������Ϊ����Ӳ�����������D��GԪ��ԭ�ӵ�������������ȡ�X��Y��Z��W���ס����������ʾ�������Ԫ�ص����ֻ�����Ԫ����ɣ�Ԫ��B�γɵĵ���M��ס��ң��ס����Ǹ��г�����Ũ�ᣩ���ܷ�Ӧ����Է���������< �ң�ת����ϵ��ͼ����Ӧ������ȥ����ԭ��E����������������Ӳ�����ȡ�Ԫ��F�γɵĵ����� ��21���͵���Դ������ĿǰӦ�����İ뵼����ϡ���ش��������⣺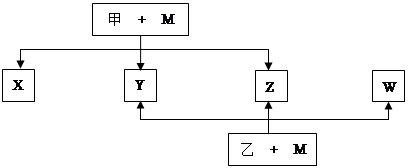
��1��A2D���۷е��A2G�ߵ�ԭ���� ��B��D��G��һ��ԭ�ӹ���ÿԭ�Ӿ�����8���ӵķ��ӣ������ʽ�� ��
��2��E4B3��ˮ��Ӧ�Ļ�ѧ����ʽ ��
��3��д��M���Ũ��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ ��
��4��X��Y��Z��W����ͬһ�����ʣ���������̬ʱ�ľ�������Ϊ ��X��Y��W������Z��Ӧ����Z�ĽṹʽΪ ��
��5����֪CH4 ��g���� 2O2��g����CO2 ��g����2H2O��l�� ��H1��a kJ/mol
�����㷴ӦCH4 ��g���� 4NO ��g����2N2 ��g����CO2 ��g����2H2O��l�����ʱ��H2 ������Ҫ����ij���Ϸ�Ӧ���ʱ��H3������Ӧ�и����ʻ�ѧ������֮��Ϊ���������ʱ ��H3 =" b" kJ/mol����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
�ݴ˼������H2 = kJ/mol���ú�a��b��ʽ�ӱ�ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�㺬�л�����B��Bֻ������Ԫ����ɣ����ĺ�������״���ֲ�����������Ӱ��ܴ�ʹ����Ӳ���࣬���˽��л�е�ӹ�����֪��(1)E��F��H��I��P������Ϊ���壬H��I��PΪ���ʣ�E��һ�ֺ���ɫ���塣(2)��Ӧ�٢ھ�Ϊ�Ʊ��������ɫˮ������Na2FeO4�ķ��������з�Ӧ��������Na2FeO4ͬʱ������NaNO2��H2O��������֮�ŵ�ת����ϵ����ͼ��ʾfͼ�в���������û���г�����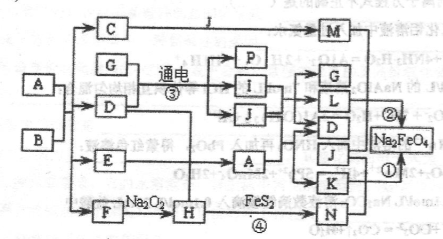
����д���¿հף�
(1)�õ���ʽ��ʾF���γɹ���_________________________________________
(2)��Ҫ��������з�Ӧ��__________________________________________
��Ӧ�۵����ӷ���ʽΪ________________________________________
��Ӧ�ٵĻ�ѧ����ʽΪ________________________________________
(3)��Ӧ�����������뻹ԭ�������ʵ�����Ϊ__________________________________
��Ӧ���е���������Ļ�ѧʽΪ_______________________________________
(4)ʵ�����м���C��Һ�н��������ӵij��÷�����________________________
(5)���A��B��Ӧʱ����E��F�����ʵ�����Ϊ13:1������B�Ļ�ѧʽΪ__________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com