
ʵ������Ҫ0.1 mol·L��1 NaOH��Һ450 mL��0.5 mol·L��1������Һ500 mL��������������Һ����������ش��������⣺
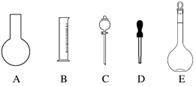
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����________________________________________________________________________
(�����)������������Һ�����õ��IJ���������______(����������)��
(2)������NaOH��Һʱ��
�ٸ��ݼ�����������ƽ��ȡNaOH������Ϊ________g��
����NaOH��Һ��ת��������ƿʱ����������������������ҺŨ��________(�>������<������)0.1 mol·L��1��
����NaOH�����ܽ��������������ƿ��ϴ�ձ���ϴ��Һ��������ƿ�����ݣ���������ҺŨ��________(�>������<������)0.1 mol·L��1��
(3)������������Һʱ��
��������������Ϊ98%���ܶ�Ϊ1.84 g·cm��3��Ũ��������Ϊ________(����������һλС��)mL��
�����ʵ������15 mL��20 mL��50 mL��Ͳ��Ӧѡ��________mL��Ͳ��ã�
�����ƹ������������ձ��н�Ũ�������ϡ�ͣ�ϡ��ʱ����������________________________________________________________________________��
�𰸡�(1)AC���ձ���������
(2)��2.0����<����>
(3)��13.6����15���۽�Ũ���������ڻ�������ˮ�У����ò��������Ͻ���
������(1)������Һ��Ҫ���ֲ�����������Ͳ���ձ�������������ͷ�ιܡ�����ƿ��
(2)������450 mL������ƿ������NaOH��ҺҪ��500 mL������ƿ��m(NaOH)��c·V·M��0.1 mol·L��1��0.5 L��40 g·mol��1��2.0 g����NaOH����ˮ�ų������ȣ�Ӧ������ȴ�����º�����������ƿ�У������ݺ���Һ��ȴ�����º������С��Ũ��ƫ�ߡ�
(3)��c(Ũ)·V(Ũ)��c(ϡ)·V(ϡ)���� ��V(Ũ)��0.5��0.5����V(Ũ)��0.013 6 L��13.6 mL����ѡ��15 mL��Ͳ��ã����С����ע�����㣺����ˮ�������ڡ������衣
��V(Ũ)��0.5��0.5����V(Ũ)��0.013 6 L��13.6 mL����ѡ��15 mL��Ͳ��ã����С����ע�����㣺����ˮ�������ڡ������衣


 ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ԭ�ӵ�������a g,12Cԭ�ӵ�������b g����NA��ʾ�����ӵ�������ֵ������˵������ȷ����(����)
A������ԭ�ӵ����ԭ������Ϊ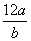
B��m g����ԭ�ӵ����ʵ���Ϊ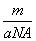 mol
mol
C������ԭ�ӵ�Ħ��������aNA g
D��a g����ԭ�������ĵ�����Ϊ17 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶��£�ij��������������Һ���ΪV mL����Һ�ܶ�Ϊd g·cm��3����������Ϊw�����ʵ���Ũ��Ϊc mol·L��1����Һ�к��������Ƶ�����Ϊm g�����¶���NaOH���ܽ��ΪS��
(1)��w����ʾ���¶����������Ƶ��ܽ��(S)Ϊ________________________________________________________________________��
(2)��c��d����ʾ���¶���NaOH���ܽ��(S)Ϊ________________________________________________________________________��
(3)��m��V��ʾ��Һ�����ʵ����ʵ���Ũ��(c)Ϊ________________________________________________________________________��
(4)��w��d��ʾ��Һ�����ʵ����ʵ���Ũ��(c)Ϊ________________________________________________________________________��
(5)��c��d��ʾ��Һ�����ʵ���������(w)Ϊ________________________________________________________________________��
(6)��S��ʾ��Һ�����ʵ���������(w)Ϊ________________________________________________________________________��
(7)��S��d��ʾ��Һ�����ʵ����ʵ���Ũ��(c)Ϊ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״����V L�����ܽ���1 Lˮ��(ˮ���ܶȽ���Ϊ1 g·mL��1)��������Һ���ܶ�Ϊ�� g·mL��1 ����������Ϊw�����ʵ���Ũ��Ϊc mol·L��1�������й�ϵ�в���ȷ����(����)
A���ѣ�(17V��22 400)/(22.4��22.4V)
B��w��17c/(1 000��)
C��w��17V/(17V��22 400)
D��c��1 000V��/(17V��22 400)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��a L Al2(SO4)3��(NH4)2SO4�Ļ����Һ�м���b mol BaCl2��ǡ��ʹ��Һ�е�SO ��ȫ���������������ǿ����ȿɵõ�c mol NH3����ԭ��Һ��Al3����Ũ��(mol·L��1)Ϊ(����)
��ȫ���������������ǿ����ȿɵõ�c mol NH3����ԭ��Һ��Al3����Ũ��(mol·L��1)Ϊ(����)
A.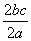 B.
B.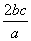 C.
C. D.
D.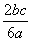
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ɵ͵��ߵ�˳��������ȷ��һ����(����)
A��1s��2p��3d��4s B��1s��2s��3s��2p
C��2s��2p��3s��3p D��4p��3d��4s��3p
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�ԭ�ӻ����ӵĵ����Ų�ʽ�������(����)
A��C��1s22s22p2
B��O2����1s22s22p6
C��Cr��1s22s22p63s23p63d44s2
D��Al3����1s22s22p6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����(����)
A�����Ӽ��������Ƿ��Ӽ�����������ܳ�
B�����Ӽ�������γɳ�ʹ���ʵ��ۡ��е����ߣ������ʵ��ܽ⡢�����Ҳ����Ӱ��
C�����»����������ͬʱ�����ڷ���֮��
D�������һ������Ļ�ѧ�������㷺��������Ȼ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)���������У����γ��������______��
A���������� B�����ȴ���
C��������̼ D������
(2)�������¼��ִ�ʩ���ٶ�ȼ��úʱ������β�����г�������������ԭú��ȼ�ϣ���ȼúʱ���������������ܿ��������Դ�������ܼ�����������Ĵ�ʩ��______��
A���٢ڢ� B���ڢۢ�
C���٢ڢ� D���٢ۢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com