
����Ŀ����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH4����Cl-��Mg2����Ba2����CO32-��SO42-����ȡ���ݸ�100mL��Һ��������ʵ�飺
��һ�ݼ���AgNO3��Һ�г����������ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ���0.08mol���塣�����ݼ�����BaCl2��Һ�õ��������12.54g������������ϴ�ӡ������������Ϊ4.66g��
��������ʵ�飬�ش��������⣺
��1���ɵ�һ�ݽ��е�ʵ���ƶϸû�����Ƿ�һ������Cl-��__��
��2���ɵڶ��ݽ��е�ʵ���֪�������Ӧ����__�������ʵ���Ũ��Ϊ__��
��3���ɵ����ݽ��е�ʵ���֪12.54g�����ijɷּ����ʵ�����__��
��4���ۺ�����ʵ�飬����Ϊ���½�����ȷ����__��
A.�û��Һ��һ������K����NH4��![]() ��CO32-��SO42-�����ܺ�Cl-����n(K��)��0.04mol
��CO32-��SO42-�����ܺ�Cl-����n(K��)��0.04mol
B.�û��Һ��һ������NH4����CO32-��SO42-�����ܺ�K����Cl-
C.�û��Һ��һ������NH4����CO32-��SO42-�����ܺ�Mg2����K����Cl-
D.�û��Һ��һ������NH4����SO42-�����ܺ�Mg2����K����Cl-
���𰸡���һ�� NH4+ 0.8mol/L 0.04mol BaCO3��0.02mol BaSO4 A
��������
�������Ӽ���ķ�����ʵ��������Һ�ʵ����Է�����Һ�д��ڵ����ӡ�
��1������̼������ӡ���������Ӷ��ܹ��������ӷ�Ӧ����̼������������������������ȷ��ԭ��Һ���Ƿ���������ӣ��ʴ�Ϊ����һ������Ϊ̼���������������dz�����
��2��������NaOH��Һ���Ⱥ��ռ���0.08mol���壬������Ϊ������˵����Һ�� һ����������ӣ�����ӵ����ʵ���Ũ��Ϊ��c��NH4+��=![]() =0.8mol/L���ʴ�Ϊ��NH4+��0.8mol/L��
=0.8mol/L���ʴ�Ϊ��NH4+��0.8mol/L��
��3��������BaCl2��Һ�õ��������12.54g������������ϴ�ӡ������������Ϊ4.66g��˵������Ϊ���ᱵ��̼�ᱵ�Ļ�������4.66gΪ���ᱵ������n��BaSO4��=n��SO42-��=![]() =0.02mol��̼�ᱵ����������Ϊ��12.54g-4.66g=7.88g������n��BaCO3��=n��CO32-��=
=0.02mol��̼�ᱵ����������Ϊ��12.54g-4.66g=7.88g������n��BaCO3��=n��CO32-��=![]() =0.04mol���ʴ�Ϊ��0.04mol BaCO3��0.02mol BaSO4��
=0.04mol���ʴ�Ϊ��0.04mol BaCO3��0.02mol BaSO4��
��4���������Ϸ�����֪���ٸ��ݵ���غ㣬�����Ϊ��n��+��=n��NH4+��=0.08mol��n��-��=2n��CO32-��+2n��SO42-��=0.12mol����һ����K+������0.04mol������Һ��һ�����ڣ�K+��NH4+��CO32-��SO42-�����ܺ���Cl-�������������ӣ������ӵ����ʵ�������0.04mol���������������ӣ������ӵ����ʵ���Ϊ0.04mol������A��ȷ���ʴ𰸰�Ϊ��A��


 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������л���Ľṹ�������йص���������ȷ����
A.������( )�Ķ��ȴ�����3�֣����ȴ���Ҳ��3��
)�Ķ��ȴ�����3�֣����ȴ���Ҳ��3��
B.�л��� (CH3)2CHC(CH3)3������Ϊ2��2��3��������
C.�������Һ�м���Ũ���������Һ�����֣������ʵ����ʷ����ı䲢����
D.��CH2(NH2)��COOH��CH3��CH(NH2)��COOH���ְ�������ˮ������������4�ֶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ù�����������أ�SOEC)��ʵ������绯ѧ��������ϩ��ԭ��ʾ��ͼ������ʾ������˵���������
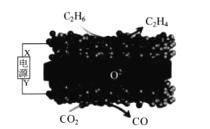
A.X �ǵ�Դ������
B.��װ���ڹ��������У�O2-�ڹ��������������·����Ϸ�Ǩ��
C.�����������ķ�ӦΪ��CO2+2e-+2H+=CO+H2O
D.�ù��̵��ܷ�Ӧ����ʽΪ��C2H6+CO2=C2H4+CO+H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������[Cr(OH)SO4]���������Ƥ���������ǿ�ȡ�һ���Ը���(��Cr2O3������Fe2O3��CaO��Al2O3��SiO2������)Ϊԭ���Ʊ�Cr(OH)SO4�Ĺ���������ͼ��

�ش��������⣺
(1)��������ʱ��������Ҫ�Ļ�ѧ����ʽΪ__��
(2)��ˮ���������У����ϵ�����(������С)�Ը���������Ӱ����ͼ��ʾ������ѷ�Ӧ����Ϊ__��
(3)������2����Ҫ�ɷ�ΪAl(OH)3��__(�ѧʽ)��������2������ҺpH����a(aС��6.5)�����������ӷ�ӦΪ__����1L������Һ�к���Ԫ�ص�����Ϊ28.6g��![]() ��
��![]() ת��Ϊ
ת��Ϊ![]() ���ữ��������Һ��c(
���ữ��������Һ��c(![]() )=__��
)=__��
(4)�����йع��ұ�����![]() �ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5.0��107mol��L1���²����ŷš���
�ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5.0��107mol��L1���²����ŷš���![]() �ķ�ˮ����ͨ�����ó���������������Ա�������BaCrO4������Ksp(BaCrO4)=1.2��1010�ݣ��ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С��__mol��L1��������ˮ�������ܴﵽ�����ŷű���
�ķ�ˮ����ͨ�����ó���������������Ա�������BaCrO4������Ksp(BaCrO4)=1.2��1010�ݣ��ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С��__mol��L1��������ˮ�������ܴﵽ�����ŷű���
(5)��֪CH3OH�����������¿ɱ���������CO2��д��Na2Cr2O7��CH3OH��Ӧ����Cr(OH)SO4�Ļ�ѧ����ʽ__��
(6)ij����m1kg�ĸ���(��Cr2O340%)�Ʊ�Cr(OH)SO4�����յõ���Ʒm2kg�������Ϊ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȡ�
��֪����CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
2Cu2����2Cl����SO2��2H2O![]() 2CuCl����4H����SO42-
2CuCl����4H����SO42-
2CuCl2��SnCl2=2CuCl����SnCl4
��CuCl2��Һ���Ҷ���(H2N��CH2��CH2��NH2)���γ������ӣ�
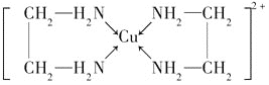
��ش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ_________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����_____________
(2)SO2���ӵĿռ乹��Ϊ_____________
(3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ_________�Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵö࣬ԭ����_____________
(4)�������γɵ��������к��еĻ�ѧ��������______(����ĸ)��
A.��λ�� B.���Լ� C.���Ӽ� D.�Ǽ��Լ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼���ʵļ�ֵ��ת��������������̼���Ϳɳ����Է�չ��������Ҫ���о���ֵ����ش��������⣺
(1)��֪CO�����л�ѧ��ΪC��O����صĻ�ѧ�������������£�
��ѧ�� | H��O | C��O | C=O | H��H |
E/(kJ��mol1) | 463 | 1075 | 803 | 436 |
CO(g)+H2O(g)CO2(g)+H2(g) ��H=__kJ��mol1��
�������������COƽ��ת���ʵĴ�ʩ��__(����)��
a.����ѹǿ b.�����¶� c.���ԭ������H2O�ı��� d.ʹ�ø�Ч����
(2)�ö��Ե缫���KOH��Һ���ɽ������е�CO2ת��Ϊ�����(HCOO)��Ȼ���һ�������Ƶ���Ҫ�л�����ԭ�ϼ��ᡣCO2������Ӧ�ĵ缫��ӦʽΪ__������������ת��1mol���ӣ�����������������(��״��)Ϊ__L��
(3)�ұ���������ȡ����ϩ�ķ�ӦΪ��
![]() (g)+CO2(g)
(g)+CO2(g)![]() (g)+CO(g)+H2O(g)���䷴Ӧ�������£�
(g)+CO(g)+H2O(g)���䷴Ӧ�������£�

��һ���¶��£�������ܱ������г���2mol�ұ���2molCO2����ʼѹǿΪp0��ƽ��ʱ���������������ʵ���Ϊ5mol���ұ���ת����Ϊ__����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��Kp=__��[�����ѹ(p��)=������ѹ(p��)�������������]
���ұ�ƽ��ת������p(CO2)�Ĺ�ϵ��ͼ��ʾ��������ұ�ƽ��ת��������p(CO2)�仯���仯��ԭ��__��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Ԫ��W��X��Y��Z��ԭ��������������W��X��Y������������֮����Z��������������ȣ�W�ļ��⻯����Z�ĵ����ڼ���ƿ�л�Ϻ����ڹ�Դ�·�����Ӧ��������ɫ�ɻ���ɫ���ϱ�dz��ƿ��������״�����ɡ�����˵��������ǣ�������
A.��ѹ��,�������ʵķе㣺![]()
B.X���ӵĵ��Ӳ�ṹ��Y���ӵ���ͬ
C.X��Z�γɵĶ�Ԫ�������ˮ��Һ������
D.Z�ֱ���W��Y�γɵĶ�Ԫ������������ѧ��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2L�ܱ������а�4molA��2molB��ϣ���һ�������·�����Ӧ3A(g)��2B(g)![]() zC(g)��2D(g)��2min��Ӧ�ﵽƽ��ʱ����1.6molC���ֲ�÷�Ӧ����v(D)=0.2mol/(L��min)��������˵����ȷ���ǣ� ��
zC(g)��2D(g)��2min��Ӧ�ﵽƽ��ʱ����1.6molC���ֲ�÷�Ӧ����v(D)=0.2mol/(L��min)��������˵����ȷ���ǣ� ��
A.z=4
B.B���ʵ�ת������20%
C.A��ƽ��Ũ����1.6mol/L
D.ƽ��ʱ����ѹǿ��ԭ����![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��Һ��
��Һ��![]() п�������ȼ��г�ַ�Ӧ����÷�Ӧǰ�¶�Ϊ
п�������ȼ��г�ַ�Ӧ����÷�Ӧǰ�¶�Ϊ![]() ����Ӧ������¶�Ϊ
����Ӧ������¶�Ϊ![]() ��
��
��֪����Ӧǰ����Һ�ı����ݾ�����Ϊ![]() ����Һ���ܶȾ�����Ϊ
����Һ���ܶȾ�����Ϊ![]() ��������Һ����������仯�ͽ������յ�����������㣺
��������Һ����������仯�ͽ������յ�����������㣺
(1)��Ӧ�ų�������![]() _____J��
_____J��
(2)��Ӧ![]() ��
��![]() ______
______![]() (��ʽ����)��
(��ʽ����)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com