
| A�� | �٢ڢۢ� | B�� | �ۢݢޢ� | C�� | �٢ۢܢ� | D�� | �ڢۢޢ� |
���� �ټ�ˮϡ�ͣ�c��H+����С��c��OH-������
�ڸ��ݻ����Һ�еĵ���غ�������غ���������
�۸���ͬŨ��ʱ�ļ���ǿ���Ƚϣ�
�����NH4NO3�����ʵ�����Ȼ�����1mol������к�2mol��ԭ����������
��pH=4.5Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ�У��������ˮ�⣻
����pH֮�͵���14���������Ͽɵ���7��
�߰�0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ����߷�����ӦNaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O����Һ�е�������NaOH��Ba��OH��2�����ߵ����ʵ���Ũ�ȷֱ�Ϊ0.05mol/L��0.1mol/L���ٽ�������غ��жϣ�
������Һ�е�������������Һ�е������Ӿ�ȫ��������ˮ�ĵ��룮
��� �⣺�ټ�ˮϡ�ͣ�c��H+����С������Kw���䣬��c��OH-�����ʢٴ���
�ڸ��ݻ����Һ�е������غ���У�2c��Na+��=3[c��CO32-��+c��HCO3-��+c��H2CO3��]�٣����������غ���У�c��Na+��+c��H+��=2c��CO32-��+c��HCO3-��+c��OH-����
���٢���ʽ�����ɵã�c��CO32-��+2c��OH-��=2c��H+��+c��HCO3-��+3c��H2CO3�����ʢ���ȷ��
�������ε�ˮ��Һ���Լ��ԣ�ͬŨ�ȣ�����ǿ��˳��Ϊd��b��c��a����pH��ȵ�������Һ���ʵ���Ũ����С����˳��Ϊd��b��c��a���ʢ���ȷ��
����Һ��NH4NO3�����ʵ���n=CV=0.1mol/L��1L=0.1mol����1mol������к�2mol��ԭ�ӣ���0.1mol������к�0.2mol��ԭ�Ӽ�0.2NA�����ʢܴ���
��pH=4.5Ũ�Ⱦ�Ϊ0.1mol•L-1��CH3COOH��CH3COONa�����Һ�У��������ˮ�⣬c��Na+����c��CH3COOH��������غ�c��CH3COO-��+c��OH-��=c��Na+��+c��H+������c��CH3COO-��+c��OH-����c��CH3COOH��+c��H+�����ʢ���ȷ��
�����ߵ�pH֮�͵���14ʱ������ҺpH=7���ʢ���ȷ��
�߰�0.1mol/L��NaHCO3��Һ��0.3mol/L��Ba��OH��2��Һ�������ϣ����߷�����ӦNaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O����Һ�е�������NaOH��Ba��OH��2
�����ߵ����ʵ���Ũ�ȷֱ�Ϊ0.05mol/L��0.1mol/L�����������غ�ã������Һ������Ũ�ȴ�С˳����c��OH-����c��Ba2+����c��Na+����c��H+�����ʢ���ȷ��
������Һ�е�������������Һ�е������Ӿ�ȫ��������ˮ�ĵ��룬��pH=3��CH3COOH��Һ��pH=11��NaOH��Һ�У�ˮ������������Ӻ���������Ϊ10-11mol/L����ˮ�ĵ���̶���ͬ���ʢ����
��ѡBD��
���� ���⿼���Ϊ�ۺϣ��漰������ʵĵ��롢pH�ļ��㡢�����ˮ���Լ�����ϵĶ����жϺͼ��㣬��Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�0.1mol Al3+���еĺ��������Ϊ0.3NA | |
| B�� | 1 mol�Ȼ��ƾ����к���NA�������� | |
| C�� | �����£�pH=12������������Һ����������������Ϊ0.2NA | |
| D�� | �����������ͭ��Һ������·��ת��NA������ʱ��������������������£�5.6L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | �� | C�� | �� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | ��������KOH | |
| B�� | ��������KOH | |
| C�� | ����H+ ͨ�����ӽ���Ĥ����ൽ�Ҳ� | |
| D�� | ����K+ͨ�����ӽ���Ĥ���Ҳൽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ܲ㺬�е�����Ϊ2n2 | |
| B�� | ���ܲ���ܼ����Ǵ�s�ܼ���ʼ��f�ܼ����� | |
| C�� | ���ܲ㺬�е��ܼ���Ϊn-l | |
| D�� | ���ܼ���ԭ�ӹ������s��p��d��f��˳������Ϊ1��3��5��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ��������ȡ | B�� | ��ȡ���������� | C�� | ��Һ���������� | D�� | ����Һ����ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2��1 | B�� | l��2 | C�� | l��1 | D�� | 3��l |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
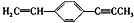 ��˵����ȷ���ǣ�������
��˵����ȷ���ǣ�������| A�� | ���ֻ����9��̼ԭ����ͬһƽ���� | |
| B�� | ��6��̼ԭ�ӿ�����ͬһֱ���� | |
| C�� | �����4��̼ԭ����ͬһֱ���� | |
| D�� | ����ԭ��һ������ͬһƽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �÷�Ӧ��ϵ��Au2O�������� | |
| B�� | Na2S4O6�ǻ�ԭ���� | |
| C�� | �÷�Ӧ��ϵ����ԭ���õ�Ԫ����Au2O��+1�۵�Au | |
| D�� | �÷�Ӧ��ϵ��ÿת��2mol����������1molH2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com